- Fourth Scientist Pleads Guilty to Stealing GSK Information for China (biospace.com)
Ex-GlaxoSmithKline researcher Lucy Xi has pled guilty to conspiracy to steal trade secrets from her former company to help a rival firm launch a business in China. She is the fourth person to plead guilty to the offense...Xi and co-defendants Yan Mei, Yu Xue, Yan Mei, and Tao Li created Renopharma in the guise of conducting research and development of anti-cancer therapies. However, it was found that Renopharma had been operating as a repository of stolen GSK information and was receiving compensation and subsidies from the People's Republic of China to do so...READ MORE
- Attorney General Aaron Ford announces Nevada to join opiod settlement (reviewjournal.com)Nevada to receive $285 million in latest round of opioid settlements (thenevadaindependent.com)
Attorney General Aaron Ford announced Thursday that Nevada would join a multi-state opiod settlement with drugmakers and distributors...Ford said that the state would receive around $285 million through a pair of settlements...Last year, Ford announced a $45 million settlement against one company involved in the opioid litigation. The lawsuit is being handled on a contingency fee basis for the state by Eglet Prince, the law firm where Ford worked as a private attorney before being elected attorney general in 2018. Ford, however, recused himself from the selection process...Ford in August announced that Nevada would opt out of a $26 billion multi-state settlement...READ MORE
- COVID-19 pills from Pfizer, Merck authorized by FDA in major pandemic milestone (biopharmadive.com)
Paxlovid and molnupiravir are the first oral treatments for COVID-19, potentially valuable new tools as the fast-spreading omicron variant fuels a sharp surge in cases across the U.S...The Food and Drug Administration...authorized the first pill for COVID-19, clearing for emergency use an antiviral treatment from Pfizer at a precarious moment in the two-year-old pandemic. One day later...the agency cleared a second pill developed by Merck & Co...READ MORE
- Moderna vaccine weaker against omicron, but third shot boosts protection (biopharmadive.com)
A booster shot of Moderna's authorized coronavirus vaccine significantly increases antibody levels against the omicron variant, according to the results of laboratory tests the company disclosed... The update comes days after another analysis indicated the initial two-shot regimen is less effective at neutralizing omicron than other variants like delta...As a result of the booster's apparent cross-protection against multiple variants, Moderna said it will focus its efforts away from a more complex "multivalent" shot and toward further study of its existing booster dose formulation. The biotech said it will still develop an omicron-specific booster, however, and aims to begin clinical testing of that in early 2022...READMORE
- Theranos founder Elizabeth Holmes guilty of fraud (healthcareitnews.com)
Holmes faced 11 total counts, and the jury found her guilty of four of them. Each carries a maximum sentence of 20 years in prison...Theranos founder and former chief executive officer Elizabeth Holmes, who once promised "lab on a chip" technology to upend the diagnostics industry...The verdict follows months of testimony, including some from doctors, former Theranos employees and board members, patients, and Holmes herself, about their experiences with the once-vaunted blood-testing startup..."Holmes and [former Theranos chief operating officer Ramesh] Balwani used advertisements and solicitations to encourage and induce doctors and patients to use Theranos' blood testing laboratory services, even though, according to the government, the defendants knew Theranos was not capable of consistently producing accurate and reliable results for certain blood tests," read the indictment...READ MORE
- Pill for treating COVID at home comes to Nevada, but in short supply (reviewjournal.com)
The first pill authorized in the U.S. for treating COVID-19 at home will initially be offered in Nevada primarily to patients in long-term care and skilled nursing facilities because of scarce supplies...The remainder, less than 10 percent of the state’s initial allotment of Pfizer’s antiviral medication Paxlovid, has been given to University Medical Center in Las Vegas and to Renown Regional Medical Center in Reno, members of the Nevada State Board of Pharmacy said...The drug’s authorization is “hands down, next to the vaccine, the most significant milestone in the pandemic,” said Dr. Shadaba Asad, the medical director of infectious disease at UMC...Supplies of the Pfizer pill currently are extremely limited across the country. Nevada’s initial supplies are enough to treat 480 patients, with allocations expected to grow as production ramps up, pharmacy board executive secretary David Wuest said...READMORE
- Nevada defends untested lethal injection plan as drugs near expiration (thenevadaindependent.com)
State corrections officials told a federal judge last week that they’re quickly running out time before a crucial lethal injection drug proposed for use in the execution of death row inmate Zane Floyd will expire...Nevada Department of Corrections Director Charles Daniels said in federal court that his chief concern with carrying out the execution is that the necessary drugs would expire before the state could proceed with its execution plan.His testimony followed days of hearings last week, during which medical experts called by the state made the case that the state’s proposed drug cocktail, never before used in carrying out a death sentence, would result in a painless death for Floyd...“My understanding is that it would be less painful than other methods,” said Daniel Buffington, a clinical pharmacologist who works at the University of South Florida. “It would reduce the individual's anxiety… It would be quick.”...
READMORE
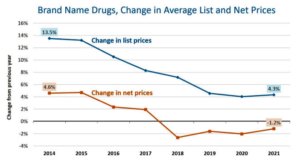
- Tales of the Unsurprised: Brand-Name Drug Prices Fell for the Fourth Consecutive Year (drugchannels.net)
Reality has again failed to cooperate with the politically motivated cries of “skyrocketing drug prices” or anecdotes about companies “jacking up prices” (as President Biden recently claimed)...Brand-name drug prices continue to decline, while the prices of other healthcare products and services continue to rise. For 2021, brand-name drugs’ net prices dropped for the fourth consecutive year. Meanwhile, brand-name drug list prices grew more slowly than overall inflation. What’s more, we project that the gross-to-net bubble for patent-protected brand-name drugs will exceed $200 billion in 2021...The factors that drive declining brand-name drug prices remain for 2022, suggesting that these trends will continue...READ MORE
- Pfizer antiviral pills may be risky with other medications (nbcnews.com)Paxlovid (drugs.com)
One of the two drugs in the antiviral cocktail could cause serious interactions with widely used prescriptions, including statins, blood thinners and some antidepressants...the first antiviral pills for Covid-19 promise desperately needed protection for people at risk of severe disease. However, many people prescribed Pfizer’s or Merck’s new medications will require careful monitoring by doctors and pharmacists, and the antivirals may not be safe for everyone, experts caution...could cause severe or life-threatening interactions with widely used medications, including statins, blood thinners and some antidepressants. And the FDA does not recommend Paxlovid for people with severe kidney or liver disease...READ MORE
- FDA enables abortion via telemedicine by lifting restrictions on pill (healthcareitnews.com)
The revised regulations mean providers can prescribe and mail abortion medication to patients without an in-person appointment in more than half the country...The U.S. Food and Drug Administration...modified its restrictions on mifepristone, one of the medications used in abortion. The new regulations enable abortion via telemedicine in more than half of the country...The permanent modification of the requirement means that abortion providers in many states will be able to send medication to patients via mail-order pharmacy, bridging some access gaps in places with few in-person clinics or burdensome multi-appointment waiting period rules...some researchers and advocates have noted that the FDA left in place some certification components for providers and pharmacies, as well as patient agreements...READMORE