- Judge conditionally approves Purdue Pharma opioid settlement, shielding Sackler family from future lawsuits (cbsnews.com)AG Morrisey arguments help end 'California Care Out' in Purdue Pharma bankruptcy case (news.yahoo.com)
... the reorganization plan conditionally approved by a federal judge Wednesday protects what some call "the most evil family in America" from any civil liability...Under the plan, the Sackler family would forfeit ownership of Purdue Pharma, turn over more than 30 million documents and pay $4.5 billion. In return, the Sackler family would be shielded from an onslaught of lawsuits. Purdue has said the settlement overall will be worth about $10 billion, which includes the value of addiction treatment and overdose antidote drugs it is developing...READ MORE
- Horse dewormer falsely believed to treat COVID-19 in short supply in Reno (rgj.com)Data Supports Use of Anti-Parasitic Drug Ivermectin in COVID-19 Patients, Study Shows (biospace.com)
Horse dewormer falsely believed to treat COVID-19 in short supply in Reno...Deworming season is fast approaching for horse owners in Northern Nevada. Every September through November, horses are administered medications containing ivermectin to stave off life-threatening parasitic intestinal worms...But this year, feed stores are having a difficult time keeping large-animal ivermectin medications on the shelves due to a spike in interest in the drug's use as a home remedy for COVID-19...READ MORE
- CMS delays enforcement of key parts of price transparency rule by 6 months (fiercehealthcare.com)
The Biden administration has delayed enforcement of key parts of a major insurer price transparency rule by six months until July 1, 2022, to give plans more time to comply...The Centers for Medicare & Medicaid Services announced the change in a new guidance released Friday focusing on the final price transparency rule released last October under the Trump administration. The guidance focuses on a requirement that certain health plans disclose online their in-network provider rates for covered items and services, out-of-network allowed amounts and billed charges for certain items and services...READ MORE
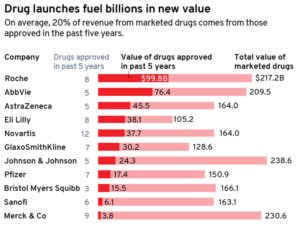
- Who’s getting the most out of their R&D engine? Pharma’s top 11, ranked (fiercepharma.com)
Drugmakers have myriad tools in their arsenal when looking to grow sales. They can acquire marketed drugs, raise prices or focus on growing the reach of their existing medicines. But it's often new drug approvals that reign supreme and ultimately prove the worth of a company's development engine...covered in a recent Evaluate Vantage report, the team at Fierce Pharma took a close look at the recent approvals for 11 of the world's biggest drugmakers by revenue. Specifically, we're highlighting the dollar value of the industry's launches from the last five years and analyzing how the new meds fit into each company's overall portfolio...READ MORE
- The FDA’s cozy relationship with Big Pharma (americanthinker.com)
No matter how you slice it, the pharmaceutical industry is the central engine of the global health establishment. The industry's larger corporations provide funding for the FDA, the CDC, the WHO; they do this both directly and through NGOs like the EPDA...The ties between the FDA and Big Pharma run deep, and their relationship has become so symbiotic that neither could exist without the other unless massive reforms were to take place. Big Pharma relies on the FDA to approve and rush its products to market, and the FDA relies on Big Pharma to receive its funding...READ MORE
- In talc case, reorg ruling goes Johnson & Johnson’s way, keeping bankruptcy in play (fiercepharma.com)
With 25,000 unresolved lawsuits alleging that its talcum products cause cancer, Johnson & Johnson is considering a legal maneuver sometimes referred to as the Texas two-step... a U.S. judge declined to block the move, giving the pharmaceutical giant the option to create a new business to absorb liabilities associated with the litigation and then seek bankruptcy protection...U.S. bankruptcy judge...denied a plaintiffs' request to issue a restraining order against J&J to prevent the company from employing the tactic, first used by firms decades ago to mitigate the costs associated with asbestos claims...READ MORE
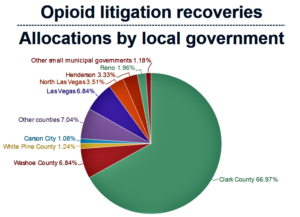
- Nevada to pursue separate opioid litigation against major drug companies; new statewide distribution plan adopted (thenevadaindependent.com)
Nevada will not sign on to a proposed $26 billion multistate settlement with the nation’s three largest drug distribution companies and drugmaker Johnson & Johnson — businesses accused of fueling the nation's opioid epidemic that has killed thousands of Nevadans — in hopes of getting a better deal...Attorney General Aaron Ford told The Nevada Independent...that the state would have received roughly $240 million from the settlements — an amount he called “woefully insufficient” — and that the state will instead pursue separate negotiations with the companies “to ensure that the people in this state are adequately recompensed for the damages that opioids have caused in our communities.”...READ MORE
- A Video History of Pharmacies and Prescription Prices: From Soda Fountains to GoodRx (drugchannels.net)
...“Why Pharmacies Overcharge,” an entertaining and provocative video on the pharmacy industry and its generic prescription pricing...It’s definitely worth your time...The video covers the history of pharmacy, from the “Soda Fountain Era” to “Lick, Stick, and Pour” to the rise of PBMs and GoodRx...READ MORE
- FDA User Fees to Rise and Fall as New Fee Agreements Move Forward (pharmtech.com)
Biopharmaceutical companies will pay more than $3 million to file an application seeking FDA approval of a new drug application or biologics license application during fiscal year 2022. This record charge to evaluate new drugs and biologics reflects agency analysis of additional personnel and resources needed to process its growing workload. The annual program fee paid by each manufacturer with marketed prescription drugs holds fairly even at about $370,000. And while the actual increase in the application fee is not that much, passing the $3 million mark has drawn attention and further concern about FDA’s overdependence on revenue from regulated industry...READ MORE
- FDA Says “Stop It” to Self-Medicating for COVID-19 with Unapproved Treatments (biospace.com)
“You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This is the Food and Drug Administration's recently tweeted advice to Americans seeking out alternative, unapproved treatments for COVID-19...The comment is aimed mainly at Mississippi residents as a follow-up to the state’s official Health Network Alert issued Friday. The Magnolia State has experienced increasing calls to its poison control line, with 70% of the calls related to the ingestion of the livestock formulation of anti-parasitic drug ivermectin...While ivermectin is FDA-approved to treat and prevent parasite infections, the drug commonly found at most local feed stores is highly concentrated for large animals such as horses and cows. In that type of formulation, it can be highly toxic to humans...READ MORE