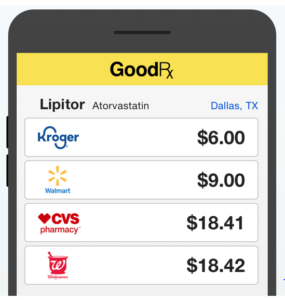
- GoodRx partners with Surescripts to provide real-time drug discount pricing (drugstorenews.com)
GoodRx has entered into an agreement with Surescripts, to deliver drug discount price information to prescribers using Surescripts Real-Time Prescription Benefit when prescribing medications for uninsured patients and patients whose price information isn’t already available from their PBM or health plan...By improving price transparency at the point of care, prescribers can have more informed conversations with their patients and ensure they can afford the treatment they need before getting to the pharmacy counter...READ MORE
- Prescription Drug Monitoring Programs: Effects on Opioid Prescribing and Drug Overdose Mortality (reason.org)PRESCRIPTION DRUG MONITORING PROGRAMS: PDMP EFFECTS ON OPIOID PRESCRIBING AND DRUG OVERDOSE MORTALITY (reason.org)
The Centers for Disease Control and Prevention reported 70,630 drug overdose deaths for 2019 in the United States, 70.5 percent of which were opioid-related...Prescription Drug Monitoring Programs are the most popular interventions states enact to address opioid addiction and overdoses...This study finds that, although Prescription Drug Monitoring Programs’ intermediary purpose to reduce prescribing has been achieved by reducing opioid distribution by 7.7 percent, they have had inconsistent effects on prescription opioid overdoses, while increasing total opioid overdoses by 17.5 percent due to increased mortality from the black market varieties by 19.8 percent...READ MORE
- Study: Drug utilization costs health industry $93B a year, with patients bearing most of the cost (fiercehealthcare.com)Quantifying The Economic Burden Of Drug Utilization Management On Payers, Manufacturers, Physicians, And Patients (healthaffairs.org)
Drug utilization management such as prior authorization and stricter formularies cost the healthcare industry $93 billion annually, with patients bearing the largest share of the cost, a new study found...The study...found that intense use of drug utilization measures has led to patients spending $35.8 billion a year in cost-sharing...“All stakeholders in the U.S. pharmaceutical system would benefit from a de-escalation of utilization management, combining lower drug prices with lower barriers of patient access,”...READ MORE
- Senate takes aim at pharma’s patent schemes, pay-for-delay deals in renewed drug pricing crackdown (fiercepharma.com)
...groups lobbying for lower prescription drug prices called on Congress to enact long-awaited reforms and stressed the urgency of the movement...Answering the call...the Senate Judiciary Committee, which voted unanimously to advance four pieces of legislation which would help rein in the cost of prescription drugs...The new laws take particular aim at the tactics used by drug companies to extend patent protections and stifle competition from cheaper generic and biosimilar drugs. The legislation, which would enhance the Federal Trade Commission's ability to initiate enforcement actions against drug companies, now moves to the Senate floor for a vote...READ MORE
- Part 1: Medicare Part B Provider Status for Pharmacists – Episode 1 (drugtopics.com)Part 2: Medicare Part B Provider Status for Pharmacists (drugtopics.com)
In an interview with Drug Topics®, Ken Perez, MBA, vice president of Healthcare Policy and Government Affairs at Omnicell, explained the crucial points of Medicare Part B provider status for pharmacists, which would allow pharmacists in underserved communities to be reimbursed for certain primary care services that they already provide in commercially ensured populations...READ MORE
- ‘Erratic’ FDA and inconsistent drug decisions put doctors off new meds: survey (fiercepharma.com)
Stunning reversals, surprise delays and controversial approvals are the order of the day at the FDA lately—and doctors have had enough...Physicians’ trust in the agency is plummeting, according to a recent survey by Spherix Global Insights. More than 40% of the doctors surveyed said their confidence in the FDA has dropped over the past year...Doctors cited a range of problems—including suspected political motivations and lack of transparency...READ MORE
- FTC drops antitrust case against AbbVie, but still decries the company’s ‘ill-gotten gains’ (fiercepharma.com)
Appealing a $448 million judgement in an antitrust case has ultimately paid off for AbbVie...the U.S. Federal Trade Commission withdrew its complaint that AbbVie used sham litigation in 2011 to illegally block generic competition for its testosterone drug AndroGel. While the agency still thinks AbbVie engaged in "anticompetitive conduct," an official said current laws don't support the agency's efforts to recoup money for consumers...READ MORE
- New database could accelerate drug repurposing for various diseases (worldpharmanews.com)
Researchers have created a new open-access database of information on drug candidates and how they are metabolised by the body, which could help speed up the repurposing of old drugs as new treatments...the process of developing new drugs is costly, can take decades, and often leads to failed treatments. The database, called NICEdrug.ch and described today in eLife, may help expedite the process by helping scientists find promising, existing drugs that might be repurposed...READ MORE
- Pfizer, Moderna and Alnylam flag pharma labor shortage in Massachusetts—and the people bottleneck doesn’t stop there (fiercepharma.com)
Raw materials and limits on high-tech equipment often take center stage when it comes to discussions around manufacturing bottlenecks. But the COVID-19 pandemic has exposed another weak link in the pharmaceutical supply chain: people...As COVID-19 vaccine production moves full-tilt, mRNA players Pfizer and Moderna are having trouble recruiting talent...Hiring challenges, which have been exacerbated by the pandemic, aren’t unique to COVID-19 vaccine makers...READ MORE
- J&J notches a win in $50M talc lawsuit, but tens of thousands still pending (fiercepharma.com)
Johnson & Johnson notched a win...with a $50 million talc lawsuit rejected in Illinois—however, tens of thousands of cases are still pending...J&J recently reported 34,600 talc-related lawsuits have now been filed...The Illinois case, filed in 2018 by relatives on behalf of Elizabeth Driscoll who died from ovarian cancer in 2016, sought up to $50 million in damages. The...jury trial found in favor of J&J, ruling the pharma giant was not liable...READ MORE