- Moderna COVID-19 Vaccine Induced Adverse Reactions In “More Than Half” Of Trial Participants (zerohedge.com)
A highly anticipated clinical trial for a potential COVID-19 vaccine managed in part by the American drug company Moderna has resulted in some adverse effects in more than half of the trial's participants, with one test group reporting "severe" symptoms...The trial, which is also being sponsored by the National Institute of Allergy and Infectious Diseases...The vaccine "induced anti–SARS-CoV-2 immune responses in all participants," the research team reported...in the New England Journal of Medicine. Researchers said that "no trial-limiting safety concerns were identified." Yet a majority of participants still reported at least one side effect..."Solicited adverse events that occurred in more than half the participants included fatigue, chills, headache, myalgia, and pain at the injection site," the report states. Fever, joint pain and nausea were also reported...READ MORE
- It’s the end of road for hydroxychloroquine in COVID-19 as Novartis, NIH and WHO pull out of trials (fiercepharma.com)
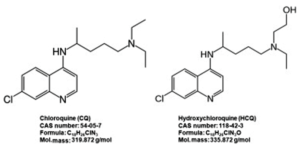
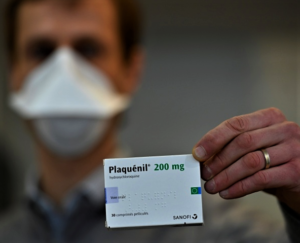
The road for hydroxychloroquine against COVID-19 is coming to an end. Three major clinical programs have been terminated after a U.K. trial found “no clinical benefit” for the malaria drug...the World Health Organization, generic hydroxychloroquine maker Novartis and the U.S. National Institutes of Health have all ended their HCQ COVID-19 studies in hospitalized patients in quick succession...The WHO and NIH cited lack of benefits for patients, while Novartis blamed “acute enrollment challenges.”...Numerous investigator-sponsored trials may still be underway, but none of them has the scale of these three to yield any convincing results...READ MORE
- Special Report: Doctors embrace drug touted by Trump for COVID-19, without hard evidence it works (reuters.com)Doctors Express Hope, Questions About Using Malaria Drugs To Combat Coronavirus (thefederalist.com)Scoop: Inside the epic White House fight over hydroxychloroquine (axios.com)
The decades-old drug that President Donald Trump has persistently promoted as a potential weapon against COVID-19 has within a matter of weeks become a standard of care in areas of the United States hit hard by the pandemic — though doctors prescribing it have no idea whether it works...Doctors and pharmacists from more than half a dozen large healthcare systems in New York, Louisiana, Massachusetts, Ohio, Washington and California told Reuters they are routinely using hydroxychloroquine on patients hospitalized with COVID-19. At the same time, several said they have seen no evidence that the drug, used for years to treat malaria and autoimmune disorders, has any effect on the virus...READ MORE
- Studies Examine Association Between Opioid Prescriptions and Obesity (drugtopics.com)
In the first study...suggested that obesity contributed significantly to incident long-term prescription opioid use...Joint pain, back pain, injury, and muscle/nerve pain were identified as the highest contributors to the excess use observed among adults with obesity...The second study...looked at the pain conditions underlying this increased likelihood of opioid prescriptions for individuals with higher BMIs...the risk of receiving prescription opioids increased progressively with BMI... Addressing the opioid crisis will require attention to underlying sources of demand for prescription opioids, including obesity, through its associations with pain...READ MORE
- Study: Hydroxychloroquine Helps Coronavirus Patients Survive (newsmax.com)Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19 (ijidonline.com)Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients (medrxiv.org)
Doctors at Henry Ford Health System in southeast Michigan said that 26% of patients who did not receive the antimalarial drug died, compared to 13% of patients who received hydroxychloroquine during their stay in the hospital...The team published their findings in the International Journal of Infectious Diseases, adding that patients who were given hydroxychloroquine alone did even better than the ones who received this drug along with azithromycin...Other studies have shown no benefits of taking hydroxychloroquine for COVID-19, and some said that it may increase the risk for cardiovascular complications...“Our results do differ from some other studies,” Dr. Marcus Zervos, division head of infectious diseases at Henry Ford, said. “What we think was important in ours is that patients were treated early. For hydroxychloroquine to have a benefit, it needs to begin before the patients begin to suffer some of the severe immune reactions that we have with COVID-19,”...READ MORE
- NIH Panel Develops COVID-19 Treatment Guidelines (drugtopics.com)NIH Covid-19 Treatment Guidelines (covid19treatmentguidelines.nih.gov)
A panel of experts convened by the US National Institutes of Health has published treatment guidelines for the coronavirus disease 2019, providing clinical recommendations for a number of therapeutic options that are currently under investigation...Importantly, the guidelines emphasize that, even though there are several therapies being tested as potential treatments, no drug has been proven to be safe and effective for treating COVID-19. Investigational antiviral agents and host modifiers and immune-based therapies were included in the guidance...READ MORE
- Using ‘Ancient History’: FDA Says Study Will Offer Plasma Therapy For COVID-19 (newsmax.com)The convalescent sera option for containing COVID-19 (jci.org)
The Food and Drug Administration on Friday announced a national study led by the Mayo Clinic that will help hospitals offer an experimental plasma therapy for COVID-19 patients, and track how they fare...The therapeutic agents—convalescent plasma and hyperimmune globulin—are both derived from the blood of people who have recovered from the disease...What the history books call “convalescent serum” was most famously used during the 1918 flu pandemic, and also against measles, bacterial pneumonia and numerous other infections before modern medicine came along...Some hospitals are already administering convalescent plasma to critical COVID-19 patients, a so-called “compassionate use” that in this case is allowed by what the FDA calls an emergency Investigational New Drug authorization...READ MORE
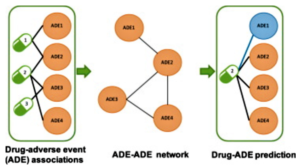
- New Machine Learning Tool Can Predict Adverse Drug Effects (drugtopics.com)Development of an adverse drug event network to predict drug toxicity (sciencedirect.com)
A new computer algorithm might be the next step toward accurate prediction of adverse drug reactions..Researchers from Harvard Medical School and the Novartis Institutes for BioMedical Research announced the creation of an open-source machine learning tool capable of predicting drug adverse effects (Aes)...The study, published in The Lancet journal EBioMedicine, examined 2 databases: 1 that reported adverse drug reactions and another with 184 proteins that specific drugs are known to interact with. Investigators constructed a computer algorithm to develop associations between the drug reactions and the 184 individual proteins...The algorithm discovered 221 associations, some known and some new. These associations indicated which proteins contribute to certain AEs and which may not...The new algorithm could help predict these AEs before the drug goes to human clinical trials, as well as before and after it enters the market...READ MORE
- Hydroxychloroquine takes another hit in failed small-scale COVID-19 study (fiercepharma.com)Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 (medrxiv.org)Hydroxychloroquine Derangement Syndrome (americanthinker.com)
Antimalarial hydroxychloroquine has raked in support as a potential wonder drug to treat COVID-19...But small-scale studies have been less than definitive on the drug's chances—and new data haven't cleared matters up much...Department of Veterans Affairs study found that severe COVID-19 patients treated with antimalarial hydroxychloroquine alone or in combination with antibiotic azithromycin showed "no evidence" of reduced risk of death or mechanical ventilation over supportive care, according to data...The researchers noted their analysis was not randomized nor controlled and cautioned patience for several ongoing clinical studies to read out before drawing conclusions on hydroxychloroquine's use for COVID-19...READ MORE
- Pharmaceutical Companies Lend Support to Hydroxychloroquine Clinical Trials for COVID-19 (pharmacytimes.com)
Pharmaceutical companies Novartis and Rising Pharmaceuticals are taking action to support the latest clinical trials exploring hydroxychloroquine as a treatment for the coronavirus disease 2019...Novartis announced March 30 that it is donating 20,000 doses of hydroxychloroquine to the University of Washington for a COVID-19 PEP clinical trial, which is expected to provide approximately 2000 patients with a 14 post-exposure regimen...Earlier in March, Novartis committed to donating up to 130 million doses, or 200mg tablets, of generic hydroxychloroquine to support COVID-19 research...Rising Pharmaceuticals has announced a collaborative agreement with the Division of Infectious Disease and International Medicine at the University of Minnesota, Department of Infectious Disease on a clinical trial investigating hydroxychloroquine as a preventive treatment for COVID-19...READ MORE