- Novavax starts late-stage trial of COVID-19 vaccine in United States (reuters.com)Novavax Announces Initiation of PREVENT-19 Pivotal Phase 3 Efficacy Trial of COVID-19 Vaccine in the United States and Mexico (ir.novavax.com)
Novavax Inc has begun a large late-stage study of its experimental COVID-19 vaccine in the United States, the drug developer said on Monday, after delaying the trial twice due to issues in scaling up the manufacturing process...It will enroll up to 30,000 volunteers across about 115 sites in the United States and Mexico, with two-thirds of them receiving the shot 21 days apart and the rest getting placebo, the company said...READ MORE
- US pays another $2B to buy more doses of Pfizer, BioNTech coronavirus vaccine (biopharmadive.com)
The U.S. government has reached a deal to acquire 100 million more doses of Pfizer and BioNTech's coronavirus vaccine, giving the U.S. enough of a stockpile to vaccinate 100 million total residents. The vaccine is administered via two shots...Under the deal, Pfizer will deliver 70 million doses by the end of June and another 30 million by July 31 The U.S. also retains an option to acquire an additional 400 million doses of the vaccine. As with the original supply deal the two struck in July, Pfizer will receive $1.95 billion, suggesting a per-dose price of about $20...READ MORE
- US close on deal with Pfizer for millions more vaccine doses (msn.com)
The U.S. government is close to a deal to acquire tens of millions of additional doses of Pfizer's vaccine in exchange for helping the pharmaceutical giant gain better access to manufacturing supplies...the deal is under discussion and could be finalized shortly...READ MORE
- FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine (fda.gov)FDA grants authorization to Moderna’s Covid-19 vaccine, the second in the U.S. (statnews.com)
Today, the U.S. Food and Drug Administration issued an emergency use authorization for the second vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The emergency use authorization allows the Moderna COVID-19 Vaccine to be distributed in the U.S. for use in individuals 18 years of age and older...READ MORE
- Pfizer, Moderna urge calm as they launch tests of vaccines against mutated COVID-19 (fiercepharma.com)
Will mRNA vaccines continue to protect the public against COVID-19 as mutant strains emerge? Pfizer and Moderna have set out to answer that question...After a mutated, fast-spreading variant of COVID-19 in the U.K. disrupted global travel over the weekend, a troubling question emerged: Will the new vaccines from Pfizer and Moderna work against this scary new strain of the virus?... They’re launching new studies designed to prove their mRNA-based shots will fend off the new coronavirus strain, while simultaneously expressing confidence this new vaccine technology is ideal for protecting against rapidly mutating viruses...READ MORE
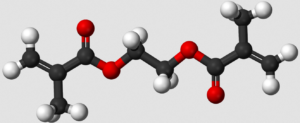
- Scientists Eye Potential Culprit Behind Covid-19 Vaccine Allergic Reactions (wsj.com)Scientists suspect compound in allergic reactions to Pfizer vaccine (axios.com)
Scientists are eyeing a potential culprit causing the allergic reactions to the Pfizer Inc. and BioNTech SE Covid-19 vaccine: the compound polyethylene glycol, also known as PEG...Six severe allergic reactions to the vaccine have been reported in the U.S., according to the Centers for Disease Control and Prevention, out of 272,001 doses administered through Dec. 19. At least two cases of anaphylaxis have also occurred in the U.K. People in the U.S. began receiving Moderna Inc.’s vaccine Monday, and no allergic reactions to it have been reported so far...READ MORE (full story behind pay wall)
- EMA authorizes Pfizer/Biontech vaccine as new SARS-CoV-2 variant emerges (bioworld.com)Comirnaty (BNT162b2) Vaccine (precisionvaccinations.com)
The EMA has issued a positive opinion on Pfizer Inc./Biontech SE’s COVID-19 vaccine, BNT-162b2, becoming the first regulator to recommend a full marketing authorization, rather than approval for emergency use...The vaccine, now brand named Comirnaty, still has to go through the formality of being approved by EU member state governments, but the EU health commissioner, Stella Kyriakides, has said she expects roll out to start on Dec. 27...“This is the first marketing authorization of a COVID-19 vaccine in the EU. It is valid in all 27 member states at the same time,” said EMA Executive Director Emer Cooke. “There is a firm scientific foundation for roll out,” she said...READ MORE
- The inside story behind Pfizer and BioNTech’s new vaccine brand name, Comirnaty (fiercepharma.com)
The new brand name for Pfizer and BioNTech's COVID-19 vaccine, Comirnaty mashes up community, immunity, mRNA and COVID—pretty much everything that could fit into the moniker for the world's most high-profile product at the moment...How did those concepts become a brand? We asked the naming agency behind both Comirnaty and its non-proprietary name, tozinameran—industry heavyweight Brand Institute, which began working with BioNTech in April. Pfizer joined the effort shortly after, when the duo’s vaccine collaboration was announced...READ MORE
- Southern Nevada Health District receives Moderna COVID vaccine (reviewjournal.com)
The Southern Nevada Health District on Tuesday received its first shipment of the Moderna COVID-19 vaccine...The initial shipment of 15,478 doses of the vaccine manufactured by Moderna will be used by the health district to vaccinate front-line health care workers, according to a statement from the agency. It comes on the heels of the 27,675 doses of the Pfizer vaccine the district already has received...Clark County hospitals also will receive shipments of 15,478 Moderna doses over the coming week...READ MORE
- FDA investigating allergic reactions to Pfizer vaccine reported in multiple states (thehill.com)Vaccinations at Chicago-area hospital to resume after 4 workers experience adverse reactions (fox32chicago.com)
The Food and Drug Administration is investigating allergic reactions to the Pfizer coronavirus vaccine that were reported in multiple states after it began to be administered this week...Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, told reporters late Friday that the reactions had been reported in more than one state besides Alaska and that the FDA is probing five reactions...“We are working hand in hand with the Centers for Disease Control and Prevention (CDC), and we’ve actually been working closely with our United Kingdom colleagues, who of course reported the allergic reaction. I think we’ll be looking at all the data we can from each of these reactions to sort out exactly what happened, and we’ll also be looking to try to understand which component of the vaccine might be helping to produce them,” Marks said...READ MORE