- 3 Coronavirus Vaccines in Phase 3 Clinical Studies: How They Stack Up Against Each Other (fool.com)
Any way you look at it, the numbers related to coronavirus vaccine candidates in development are impressive. In less than nine months, researchers have advanced 180 experimental coronavirus vaccines at least into preclinical testing. Thirty-five of those candidates are now in clinical studies...The field narrows considerably, though, when we look only at vaccine candidates that have made it to late-stage testing. Currently, only three experimental coronavirus vaccines are in phase 3 clinical trials being conducted in the U.S.:
-AstraZeneca
-Pfizer
-ModernaHere's how these three coronavirus vaccines in phase 3 testing stack up against each other...READ MORE
- CDC Head and Trump Spar Over COVID-19 Vaccine Timeline (biospace.com)
Robert Redfield, director of the U.S. Centers for Disease Control and Prevention, testified...before the U.S. Senate...He noted that although a vaccine against COVID-19 will likely be available and to begin dosing in November or December of this year, it will be limited. Getting the entire U.S. population vaccinated will likely take “six to nine months.”...He also said that face masks are “the most important, powerful public health tool we have.” He added, “I might even go so far as to say that this face mask is more guaranteed to protect me against COVID than when I take a COVID vaccine.”...READ MORE
- Researchers identify nanobody that may prevent COVID-19 infection (phys.org)An alpaca nanobody neutralizes SARS-CoV-2 byblocking receptor interaction (nature.com)
Researchers at Karolinska Institutet in Sweden have identified a small neutralizing antibody, a so-called nanobody, that has the capacity to block SARS-CoV-2 from entering human cells. The researchers believe this nanobody has the potential to be developed as an antiviral treatment against COVID-19...READ MORE
- Costa Rica researchers to trial coronavirus treatment from horse antibodies (reuters.com)Costa Rica researchers to trial coronavirus treatment from horse antibodies (scientificamerican.com)
Researchers in Costa Rica are due to begin trials of an inexpensive coronavirus treatment based on antibodies taken from horses injected with the SARS-Cov-2, the virus that causes COVID-19...Developed by University of Costa Rica’s Clodomiro Picado Institute, the equine antibodies medication is to be tested on 26 patients from mid-September...Costa Rican authorities hope to be able to begin applying the treatment more widely in hospitals if the results from the phase 2 study are encouraging. There are 471 hospitalized coronavirus patients in Costa Rica...Similar efforts are also underway in Argentina and Brazil, while scientists in Belgium are using llamas...READ MORE
- Tech startups developing rapid COVID-19 tests to be taken completely at home (fiercehealthcare.com)
Computer vision company Gauss teamed up with biotechnology company Cellex to develop a rapid, at-home and point-of-care COVID-19 antigen test...While there are other at-home COVID-19 diagnostic tests available, at least two companies are working on rapid antigen tests that can be performed by people at home without involving a laboratory...The Gauss and Cellex test has not yet gained Food and Drug Administration approval. The companies are pushing for FDA Emergency Use Authorization some time this fall...READ MORE
- A vaccine alone won’t stop Covid-19. We also need a trusted plan for it (statnews.com)
Safe and effective vaccines represent the most effective way to restore the health and economic security disrupted by the Covid-19 pandemic. To help achieve that goal, the U.S. government launched Operation Warp Speed...to accelerate development and manufacturing of several Covid-19 vaccines, with a goal of having 300 million doses available to the U.S. population by January 2021...Operation Warp Speed is expediting vaccine development primarily by moving clinical trials forward without pauses between phases, and by scaling up manufacturing capacity before knowing if a candidate works...Covid-19 vaccines can help stop the pandemic only if people trust them and want to be vaccinated. To earn and keep the trust of the American people, our government needs to ensure three key needs are met before launching any immunization campaign...READ MORE
- Ensure transparency and confidence in FDA decisions
- Ensure robust active safety monitoring as Covid-19 vaccines are rolled out
- Ensure the distribution and administration of Covid-19 vaccines are equitable and well-executed
- NIH panel says data doesn’t support plasma use for COVID-19 (biopharmadive.com)
A panel of advisers for the National Institutes of Health was not convinced convalescent plasma should be used to treat COVID-19, a recommendation that appears to conflict with a controversial decision by the Food and Drug Administration last week to issue an emergency authorization for the blood-derived treatment...The panel...reviewed the same data cited by the FDA, but concluded it to be "insufficient" to recommend "either for or against the use of convalescent plasma for the treatment of COVID-19."...the same panel cautioned against using hydroxychloroquine in treating coronavirus disease three weeks after the FDA cleared emergency use of the malaria pill...READ MORE
- CVS aims to have 4,000 drive-thru testing sites open by mid-October (fiercehealthcare.com)
CVS Health is planning to double the number of its drive-thru testing sites by mid-October, the healthcare giant announced...CVS intends to add more than 2,000 sites at its pharmacies in the next several weeks, bringing its total to more than 4,000 nationwide. The new locations will be opened in waves, beginning with 400 new sites opening on Friday...READ MORE
- Generic Drug Shortage Solutions on the Horizon (drugtopics.com)
Shortages of vital generic drugs—particularly since the start of the coronavirus disease 2019 pandemic in the US—are well known by pharmacy teams in both health systems and community settings...although pharmacy associations appreciate the Trump administration’s efforts to move more pharmaceutical and active pharmaceutical ingredient manufacturing to the United States...they say government initiatives must include an overall plan for drug pricing, payment model, and supply chain transparency...READ MORE
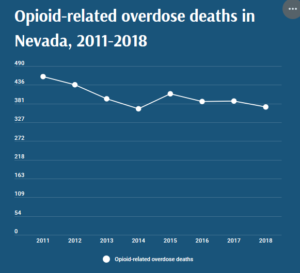
- With opioid-related overdoses on the rise, health care providers try preparing everyday Nevadans to respond to a crisis (thenevadaindependent.com)
With opioid-related overdoses on the rise, health care providers try preparing everyday Nevadans to respond to a crisis..From January to May 2020, Nevada saw 23 percent more opioid-related overdose deaths than during the same period in 2019, and similar trends are being seen across the country...Opioid-related overdose deaths peaked in Nevada in 2011 and have been on the decline since then, but around the U.S., rates have been rising throughout the COVID-19 pandemic. The American Medical Association released a report in mid-August citing news reports from 40 states and Washington, D.C. showing a rise in overdoses and illicit substance abuse since March...According to data from the Nevada Overdose Data to Action Program, there have been 197 opioid-involved drug overdose deaths in 2020 as of May 31, a 23 percent increase over the 160 counted in the first five months of 2019. April and May had the highest rates of overdose-related emergency room visits, with a 25 percent increase over the three months prior...READ MORE