- Nevada’s already slim physician workforce may grow slimmer with patients slow to return to doctor’s offices (thenevadaindependent.com)
A majority of Nevada doctors believe they can only keep their doors open for another two to six months unless the volume of patients trickling back into their offices significantly increases, according to a new survey from the American Medical Association...Ten percent of physicians in Nevada reported layoffs, 15 percent reported pay cuts, 20 percent reported temporary furloughs and 30 percent reported a reduction in staff hours, while 55 percent reported none of those changes, according to preliminary results from the survey, which Dr. Ron Swanger, president of the Nevada State Medical Association, presented to the Patient Protection Commission...READ MORE
- FDA Publishes Guidance on CGMP Requirements During COVID-19 (pharmtech.com)
FDA published guidance on June 19, 2020 detailing the agency’s recommendations for current good manufacturing practices (CGMP) requirements for addressing COVID-19 infection in employees engaging in drug manufacturing. The guidance was issued to help mitigate and prevent effects on drug safety and quality by employees confirmed to be either infected with COVID-19 or potentially exposed to someone with COVID-19...READ MORE
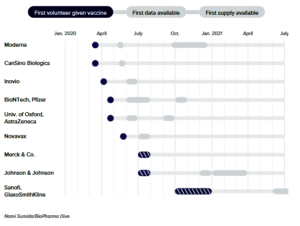
- The coronavirus vaccine frontrunners have emerged. Here’s where they stand
Fast progress by several companies has spurred hopes that a vaccine is coming soon, spurring jockeying among governments to secure supplies…Scientists, drugmakers and governments are moving with unprecedented speed to deliver a vaccine to protect against the new coronavirus…The fastest of them have already delivered preliminary data from human studies, and further results from others should come quickly as the year progresses…The goal, at least in the U.S., is to have a vaccine ready for use in some fashion by the end of the year, or early next. Doing so would be a scientific feat with few parallels. No vaccine has ever been developed so quickly, never mind manufactured for the world…Vaccine frontrunners plan for fast development…READ MORE
- FDA Releases Drug Interaction Warning for Remdesivir and Hydroxychloroquine (drugtopics.com)
The FDA released a warning to health care providers concerning an update on potential drug reactions for remdesivir, an antiviral drug that is being evaluated as a potential treatment for the novel coronavirus disease 2019 and has also been granted emergency use authorization status for treating hospitalized patients with severe COVID-19...The FDA is also revising their fact sheet for health care providers to include the warning that co-administration of remdesivir and chloroquine phosphate or hydroxychloroquine sulfate may result in less effective antiviral activity of remdesivir. The revised fact sheet also clarifies dosing and administration recommendations, and will provide additional safety data and updates from clinical trials from the National Institutes of Health and Gilead Sciences, Inc, the company that sponsors the drug and has donated 607,000 vials of remdesivir to the United States government...READ MORE
- New fair price for Gilead’s remdesivir? Below $2,800 if dexamethasone lives up to its COVID-19 promise (fiercepharma.com)
Gilead Sciences' remdesivir should be priced at no higher than $2,800 if peer-reviewed dexamethasone data support the steroid as the new COVID-19 standard of care, the Institute for Clinical and Economic Review says...Nearly two months ago, an influential drug cost watchdog pegged $4,460 as the fair price for Gilead Sciences’ authorized COVID-19 therapy remdesivir. But on coronavirus time, that's an eternity—and a lot has changed since then...In an updated assessment...the Institute for Clinical and Economic Review slightly dialed up its cost-effective price for remdesivir to a range of $4,580 to $5,080 based on detailed clinical data, updated cost estimates and interactions with Gilead...A recent announcement from U.K. researchers on the successful use of low-cost dexamethasone in a large COVID-19 clinical trial added another wrinkle to the price. That is, if the steroid’s benefits are confirmed in a peer-reviewed paper and therefore qualify it as the new standard of care, remdesivir’s cost should be cut to around $2,520 to $2,800, ICER said...READ MORE
- It’s the end of road for hydroxychloroquine in COVID-19 as Novartis, NIH and WHO pull out of trials (fiercepharma.com)
The road for hydroxychloroquine against COVID-19 is coming to an end. Three major clinical programs have been terminated after a U.K. trial found “no clinical benefit” for the malaria drug...the World Health Organization, generic hydroxychloroquine maker Novartis and the U.S. National Institutes of Health have all ended their HCQ COVID-19 studies in hospitalized patients in quick succession...The WHO and NIH cited lack of benefits for patients, while Novartis blamed “acute enrollment challenges.”...Numerous investigator-sponsored trials may still be underway, but none of them has the scale of these three to yield any convincing results...READ MORE
- Pharmacy Groups Praise New York COVID-19 Pharmacist Vaccination Law (drugtopics.com)
New York pharmacists can provide the coronavirus disease 2019 vaccine when it becomes available, according to new legislation...The Community Pharmacy Association of New York State and NACDS praised the new law, based on legislation (S. 8182-A / A. 10508-A) that adds COVID-19 to the list of illnesses for which pharmacists can vaccinate...The legislation was...was signed into law by New York Governor Andrew Cuomo...“Now is the time to make sure that people will be able to get their COVID-19 vaccination as soon as it becomes available. It is essential that all states continue to remove barriers for pharmacies to help meet the needs of patients during this phase of the pandemic,”...READ MORE
- NIH launches analytics platform to harness nationwide COVID-19 patient data to speed treatments (nih.gov)
The National Institutes of Health has launched a centralized, secure enclave to store and study vast amounts of medical record data from people diagnosed with coronavirus disease across the country. It is part of an effort, called the National COVID Cohort Collaborative (N3C), to help scientists analyze these data to understand the disease and develop treatments. This effort aims to transform clinical information into knowledge urgently needed to study COVID-19, including health risk factors that indicate better or worse outcomes of the disease, and identify potentially effective treatments...READ MORE
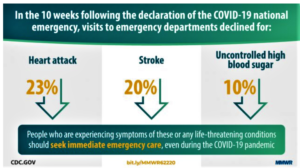
- U.S. emergency visits due to heart attacks fell during early days of COVID-19 (reuters.com)Potential Indirect Effects of the COVID-19 Pandemic on Use of Emergency Departments for Acute Life-Threatening Conditions — United States, January–May 2020 (cdc.gov)
Fewer Americans were admitted to emergency departments with life-threatening conditions such as heart attacks during the initial months of the COVID-19 pandemic,..The study suggests that patients may be delaying or avoiding seeking care because of fear of COVID-19, researchers from the U.S. Centers for Disease Control and Prevention said...study...showed that the number of deaths in New York City from causes other than COVID-19 rose by more than 5,000 people above the seasonal norm during the first two months of the pandemic...Visits to the emergency department because of heart attacks fell 23%, ten weeks after the pandemic was declared a national emergency, compared with ten weeks before the emergency declaration...READ MORE
- OHSU’s COVID-19 Study Accused Of Racial Bias (opb.org)
Charges of racial bias in the design of an Oregon study of COVID-19’s spread are raising questions about whether it will do anything to help Black and Latino communities, which have been among those hardest hit by the pandemic...“All it will be able to say is if white people are fine. And then we open up counties and people of color will die,” said Andres Lopez, research director for the Coalition of Communities of Color, a Portland-based alliance of organizations representing a number of different communities of color...The Key to Oregon Study, which plans to enlist 100,000 Oregonians and monitor them for a year for COVID-19 symptoms, will include what its designers are calling “a focus on enrolling people who fully represent the state, including our diversity in geography, socioeconomic status and communities of color.”...critics doubt Key to Oregon will succeed in its goal. They say the study design is fundamentally flawed, and that those flaws could have been avoided if people of color had been brought to the table when the study was being created...READ MORE
-Responding with listening sessions
-Advocates say study design suppresses Black and Latino voices
-OHSU methodology overlooks lessons of the past
-The principle of ‘nothing about us without us’
-OHSU researchers respond