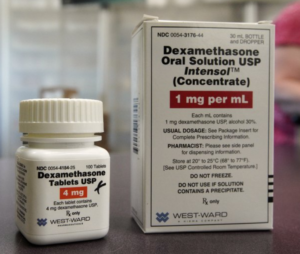
- Cheap drug is first shown to improve COVID-19 survival (apnews.com)
Researchers in England say they have the first evidence that a drug can improve COVID-19 survival: A cheap, widely available steroid reduced deaths by up to one third in severely ill hospitalized patients...The results were announced Tuesday and the British government immediately authorized the drug’s use across the United Kingdom for coronavirus patients like those who did well in the study. Researchers said they would publish results soon in a medical journal, and several independent experts said it’s important to see details to know how much of a difference the drug, dexamethasone, might make and for whom...READ MORE
- UK begins coronavirus vaccine trial; France pledges funding (apnews.com)
Scientists at Imperial College London will start immunizing people in Britain this week with their experimental coronavirus shot, while pharmaceutical company Sanofi and the French government announced more than 800 million euros ($890 million) in investment...as part of the worldwide race to find an effective vaccine...About a dozen vaccine candidates are currently in early stages of testing in thousands of people. There are no guarantees any will work but there’s increasing hope that at least some could be ready by the end of the year...the British government said 300 healthy people will be immunized with two doses of the COVID-19 vaccine candidate developed at Imperial, which has been backed by 41 million pounds ($51 million) in government funding...READ MORE
- Accuracy Still Unknown for Many Coronavirus Tests Rushed Out (newsmax.com)
How accurate are the coronavirus tests used in the U.S.?...Months into the outbreak, no one really knows how well many of the screening tests work, and experts at top medical centers say it is time to do the studies to find out...The FDA said in a statement that it has already asked multiple test makers to do follow-up accuracy studies, although it didn’t say for how many of the more than 110 authorized screening tests. The agency also said it is tracking reports of problems. Accuracy has also been an issue with blood tests that look for signs of past infections...the FDA warned doctors of a potential accuracy problem with Abbott Laboratories’ rapid ID Now test, which delivers results in roughly 15 minutes...READ MORE
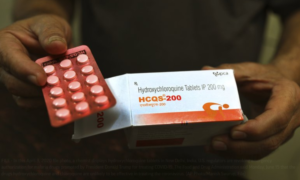
- The Lancet’s COVID Fiasco (hoover.org)
On June 4...The Lancet, a venerable British medical publication, formally retracted a thoroughly flawed study on the drug hydroxychloroquine, originally published on May 22...The key findings of the study were stunners: most critically, that the use of HCQ led to a substantial increase in mortality rates—around 30 percent—and the occurrence of cardiac arrhythmias in these COVID-19 patients...Many experts immediately promoted the study...the initial burst of favorable publicity did not prevent a large cadre of researchers in the field from questioning its findings...pointing out multiple defects...The retraction brings into question The Lancet’s editorial process. All of the defects pointed out in the open letter should have been evident to the editors before the study was published, yet at no point did The Lancet’s retraction explicitly acknowledge any breakdown in its internal procedures. It has been hinted more than once that The Lancet took its hasty position in part because of its own visceral distaste for President Donald Trump, who has long been derided for his advocacy of HCQ to ward off the virus. Indeed, on May 16, 2020, The Lancet published an editorial calling for his defeat in the 2020 Presidential election...READ MORE
- Show me the data: U.S. doctors skeptical of reported COVID breakthrough (reuters.com)
The report on...a powerful treatment for the new coronavirus brought skepticism along with optimism among U.S. doctors, who said the recent withdrawal of an influential COVID-19 study left them wanting to see more data...Global pressure to find a cure or vaccine has accelerated the process of reporting coronavirus study results, feeding confusion over whether therapies have been proven effective. One influential COVID study was withdrawn this month by respected British medical journal The Lancet over data concerns...Researchers in Britain said dexamethasone, used to fight inflammation in other diseases, reduced death rates of the most severely ill COVID-19 patients by around a third, and they would work to publish full details as soon as possible...But hours later South Korea’s top health official cautioned about the use of the drug for COVID-19 patients due to potential side effects...READ MORE
- NHS waiting list will more than double to 10 million by Christmas, health experts warn (standard.co.uk)
Around 10 million people will be on the waiting list for NHS treatment by the end of the year, more than double the current figure, health bosses have warned...Projections show the combined effects of keeping up social distancing, the backlog of treatments and challenges around staffing mean the list could rise from 4.2 million currently to around 10 million by Christmas...This is the most realistic scenario, and assumes the health service making a steady return to full capacity within the next 12 months...The pessimistic scenario, according to the NHS Confederation, assumes a second wave of Covid-19 and a lack of treatments or a vaccine, pushing the waiting list to around 11 million by the end of the year...The confederation, which represents health and care leaders, published a new report warning that the health service in England "faces an uphill battle" as it continues to manage thousands of sick and recovering Covid-19 patients while also trying to restart services such as those for cancer, stroke and heart disease...READ MORE
- US revokes emergency use of malaria drugs vs. coronavirus (apnews.com)
U.S. regulators on Monday revoked emergency authorization for malaria drugs...amid growing evidence they don’t work and could cause serious side effects...The Food and Drug Administration said the drugs hydroxychloroquine and chloroquine are unlikely to be effective in treating the coronavirus. Citing reports of heart complications, the FDA said the drugs’ unproven benefits “do not outweigh the known and potential risks.”...The move means that shipments of the drugs obtained by the federal government will no longer be distributed to state and local health authorities for use against the coronavirus. The drugs are still available for alternate uses, so U.S. doctors could still prescribe them for COVID-19 — a practice known as off-label prescribing...READ MORE
- EU calls for global alliance to buy COVID-19 vaccines up front (reuters.com)
The European Commission called...for global leaders to cooperate to buy bulk quantities of potential COVID-19 vaccines, to avoid “harmful competition” in the race for a shot and ensure any future vaccine is available for poor countries...With around a dozen potential vaccines now in human trials, rich countries have been rushing to buy up doses in advance from pharmaceutical companies, to make sure they will have enough supply should any prove successful...The European Commission, the EU executive arm, is worried that such competition could raise the prices of vaccines for everyone, and also leave many countries, mostly poor ones, struggling to obtain a supply...READ MORE
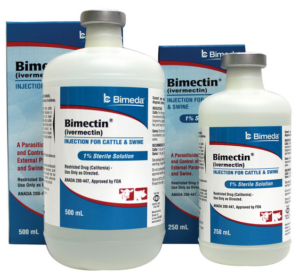
- Peru Embraces Ivermectin as Key Drug to Fight Coronavirus (newsmax.com)How a Grass Roots Health Movement Led to Acceptance of Ivermectin as a COVID-19 Therapy in Peru (trialsitenews.com)
Peru is emerging as a leader in using an anti-parasitic drug to treat COVID-19 patients...doctors...are employing the use of ivermectin in the fight against the coronavirus...As word spread in the medical community that ivermectin was showing promise in successfully treating people afflicted with COVID-19, the Peruvian government approved its use for the disease in early May — without conducting any trials or having direct, firsthand evidence of its success. TrialSite News reported that Peru is now attempting to secure almost 500,000 treatments of the drug that's normally used to kill head lice and other parasites...READ MORE
- FDA cracks down on online retailers selling scam COVID-19 treatments (mmm-online.com)Fraudulent Coronavirus Disease 2019 (COVID-19) Products (fda.gov)
With little still known about the novel coronavirus and its treatments, some shady websites are grabbing coronavirus-related URLs to push unproven products to consumers...The Food and Drug Administration has issued more than 20 warning letters since mid-May to websites selling unproven COVID-19 treatments. These online retailers are selling products like hydroxychloroquine, essential oils and alternative sanitizers that claim to treat COVID-19 or kill the virus on surfaces...The FDA took action against these websites, requiring that they remove claims that certain products will treat COVID-19 and stop selling drugs like chloroquine for unapproved uses...READ MORE