- Nevada hospital reports kept secret amid coronavirus spread (reviewjournal.com)
The Nevada Hospital Association has reportedly threatened to stop providing state health officials with daily reports detailing acute-care hospitals’ coronavirus activity, if those officials share the information with the public...For almost four weeks, the Review-Journal has sought copies of the documents from state and local governments under Nevada’s Public Records Act in order to provide the information to the public. The NHA has refused to provide the reports, which are provided to Gov. Steve Sisolak and other top state officials as they make critical decisions during the COVID-19 outbreak...While the hospital association is a private nonprofit that is not bound to respond to record requests, (Patrick) File (Nevada Open Government Coalition president) said the daily reports became subject to the state’s public records act as soon as the government received copies...READ MORE
- Nearly a dozen approved drugs could be effective against COVID-19: study (reuters.com)
At least 10 different drug compounds ranging from cancer therapies to antipsychotics and antihistamines may be effective at preventing the new coronavirus from multiplying in the body, according to a multidisciplinary study conducted by a team of scientists in the United States and France...The researchers mapped the human proteins the virus interacts with inside the body when it infects cells and makes copies of itself, then looked for compounds that could block the virus from using those proteins...The result showed that 47 compounds in cell cultures had the desired effect, at least 10 of which are already in approved drugs or being studied for diverse conditions, but could be repurposed against COVID-19, the illness caused by the new coronavirus...READ MORE
- Quest Diagnostics launches first consumer-ordered COVID-19 antibody test (fiercehealthcare.com)
For about $120, anyone can get now get a COVID-19 antibody test from Quest Diagnostics...The lab company announced Tuesday it launched the first consumer-ordered test—yes, that means there's no doctor referral needed—to allow patients to check whether they have the antibodies that indicate they've had the novel coronavirus and may have some immunity to it...The test will be available through QuestDirect, which is Quest's consumer-initiated testing business. Antibody testing uses blood serum specimens and is sometimes referred to as serology testing...READ MORE
- Trump cuts U.S. research on bat-human virus transmission over China ties (politico.com)Why US outsourced bat virus research to Wuhan (asiatimes.com)
The National Institutes of Health on Friday told EcoHealth Alliance, the study’s sponsor for the past five years, that all future funding was cut...The Trump administration abruptly cut off funding for a project studying how coronaviruses spread from bats to people after reports linked the work to a lab in Wuhan, China, at the center of conspiracy theories about the Covid-19 pandemic’s origins...The National Institutes of Health on Friday told EcoHealth Alliance, the study’s sponsor for the past five years, that all future funding was cut. The agency also demanded that the New York-based research nonprofit stop spending the $369,819 remaining from its 2020 grant...“At this time, NIH does not believe that the current project outcomes align with the program goals and agency priorities,” Michael Lauer, the agency’s deputy director for extramural research...READ MORE
- FDA Issues Emergency Use Authorization for Remdesivir in COVID-19 (drugtopics.com)1FACT SHEET FOR HEALTH CARE PROVIDERSEMERGENCY USE AUTHORIZATION (EUA) OF REMDESIVIR (GS-5734™) (fda.gov)Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment (fda.gov)
Officials with the FDA have issued an emergency use authorization for remdesivir for the treatment of suspected or laboratory-confirmed coronavirus disease 2019 in hospitalized adults and children, according to a press release...The EUA allows for remdesivir to be distributed in the United States and administered intravenously by health care providers, as appropriate, to treat suspected or laboratory-confirmed COVID-19...Evaluation of EUA criteria and scientific evidence indicate that remdesivir may be effective in treating the virus, for which there are currently no FDA-approved therapies. According to the FDA, “given there are no adequate, approved, or available alternative treatments, the known and potential benefits to treat this serious or life-threatening virus currently outweigh the known and potential risks of the drug’s use.”...READ MORE
- Southern Nevada hospitals to resume elective surgeries next week (reviewjournal.com)
Southern Nevada’s major hospitals plan to resume “medically necessary” elective surgeries and procedures Monday, according to a Nevada Hospital Association letter...The letter, dated Tuesday, was sent this week to medical staff at University Medical Center, North Vista Hospital and The Valley Health System, Dignity Health and HCA Healthcare hospital systems. The companies own and operate more than a dozen local hospitals that have more than 4,000 staffed acute-care beds...Scheduling elective surgeries and procedures can begin immediately...READ MORE
- Gov. Sisolak says some necessary medical, dental procedures may go forward (thenevadaindependent.com)Sisolak loosens restrictions on golf, drive-in worship services May 1; other elements of stay-at-home order extended (thenevadaindependent.com)Business leaders slam Sisolak on slow reopening plans (reviewjournal.com)
Gov. Steve Sisolak announced late Tuesday that the Nevada Hospital Association was preparing to resume some “medically necessary” elective procedures in the coming days — the first sign that some restrictions put in place to slow the spread of COVID-19 may be eased in the coming weeks...Unlike many other industries that were temporarily closed by emergency order, Sisolak never issued an order legally curtailing such procedures. State hospitals had instead sought to postpone unnecessary hospital visits on their own, especially as they geared up for an influx of coronavirus infections during March...READ MORE
- COVID-19 restrictions send generic drug shipping costs through the roof: survey (fiercepharma.com)
Global restrictions spawned by the coronavirus pandemic have sent transport costs skyrocketing for generics and biosimilar makers, a trade association finds. But despite the dire report, COVID-19 might not be all bad news for the industry...Average shipping costs have jumped by 224% as the pandemic added new kinks to the global drug supply chain, a survey from the Association for Accessible Medicines found..."The global pharmaceutical ecosystem is built on a highly complex supply chain,” AAM interim CEO Jeff Francer said in a release. “This ongoing crisis illustrates the importance of developing new strategies and policies that enhance the pharmaceutical supply chain in the U.S. and increase our nation’s self-sufficiency.”...READ MORE
- A 1st: US study finds Gilead drug works against coronavirus (apnews.com)Data on Gilead drug raises hopes in pandemic fight, Fauci calls it 'highly significant' (reuters.com)Adaptive COVID-19 Treatment Trial (ACTT) (clinicaltrials.gov)Analysts to Gilead CEO: What's your plan to monetize remdesivir? (fiercepharma.com)
For the first time, a major study suggests that an experimental drug works against the new coronavirus, and U.S. government officials said Wednesday that they would work to make it available to appropriate patients as quickly as possible...In a study of 1,063 patients sick enough to be hospitalized, Gilead Sciences’s remdesivir shortened the time to recovery by 31% — 11 days on average versus 15 days for those just given usual care, officials said. The drug also might be reducing deaths, although that’s not certain from the partial results revealed so far...Remdesivir was being evaluated in at least seven major studies, but this one, led by the NIH, was the strictest test. Independent monitors notified study leaders just days ago that the drug was working, so it was no longer ethical to continue with a placebo group...READ MORE
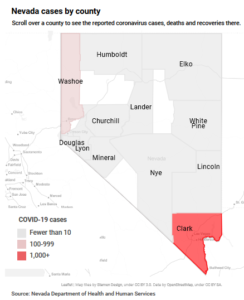
- Coronavirus in Nevada -Tracking the spread through data (reviewjournal.com)
COVID-19 has killed more than 200 people in Nevada...Most of those who died have been age 65 or older, but a handful of young adults have succumbed to the disease as well...In Clark County, COVID-19 has killed black and Asian residents at a disproportionately high rate compared to their white and Hispanic counterparts...The state’s first day of double-digit fatalities occurred in early April...READ MORE