- Federal government approves Nevada’s request for major disaster declaration, allowing access to additional resources (thenevadaindependent.com)Sisolak: More PPE, tests needed to combat COVID-19, state may need stricter enforcement of social distancing (thenevadaindependent.com)
Nevada’s request for a major disaster declaration, opening up additional paths for federal assistance under the national emergency proclamation, has been approved, Gov. Steve Sisolak announced Saturday afternoon...The declaration, which was requested by the state on Tuesday, will allow federal dollars to flow to Nevada to support Carson City and local governments’ ongoing responses to the COVID-19 pandemic. Federal funding is expected to be made available to governmental agencies and certain private nonprofit organizations for emergency protective measures...READ MORE
- Unlike FDA, European regulators refuse to clear chloroquine for COVID-19 without data (fiercepharma.com)
...days after the FDA gave them (chloroquine and hydroxychloroquine) an emergency approval to treat COVID-19...European regulators are limiting their COVID-19 use to clinical trials only...The decision comes as limited data—some of it questionable—rolls in about the drugs and their potential as COVID-19 therapies. A French study that's made headlines continues to draw fire, but brand-new data from China add to the positive case...In guidance...the European Medicines Agency restricted general use of the drugs—already approved to treat malaria and autoimmune diseases—to patients taking them for approved indications. COVID-19 patients can receive the drugs as part of clinical trials or through national emergency use programs...READ MORE
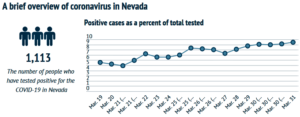
- Coronavirus contextualized: Exploring, through data, COVID-19 in Nevada and beyond (thenevadaindependent.com)
There are lots of numbers swirling around in the time of coronavirus: Confirmed cases of COVID-19, people tested, number of hospitalizations and, increasingly, new deaths...There are also other data points revealing the finer points of how the virus is affecting people, such as the age and gender of those who have tested positive and what pre-existing conditions people hospitalized after contracting the novel coronavirus have...But those numbers can be difficult to parse without context. Below, The Nevada Independent explores that data and puts it into context, walking through what we do and don’t know about coronavirus in Nevada, how Nevada stacks up against other states and projections for the future...READ MORE
- Coronavirus (COVID-19) Update: FDA takes further steps to help mitigate supply interruptions of food and medical products (fda.gov)
During this COVID-19 pandemic, the FDA is working around the clock to make sure that Americans have access to safe food and medical products. The agency is continuously examining the global supply chain to identify any concerns and assess the availability of the products Americans need most. We are also partnering with the Federal Emergency Management Agency (FEMA) on supply chain issues, including importation of needed medical products to support the U.S. response. Here is a status update and details on some of the latest actions we have taken:...READ MORE
Medical Devices
Human Drugs & Biologics and Animal Drugs
Blood Supply
Human and Animal Food
Veterinary Medicine
- Greece suggests EU buy patent rights for vaccines and coronavirus tests: FAZ (reuters.com)
Greece has suggested EU member states jointly buy patent rights for vaccines against COVID-19 and rapid tests under development to help ensure that if they are effective they are quickly distributed to those in need across the bloc...Greek Prime Minister Kyriakos Mitsotakis said finding a solution for a rapid distribution of vaccines, when they are available, is difficult but also urgent...At least 20 vaccines against COVID-19 are under development, many of which are subsidised by individual governments or charities...“Ideally, once their efficacy has been proven, such vaccines should be distributed as quickly and fairly as possible, and at a reasonable cost,”...Purchasing such patent rights would give global pharmaceutical companies incentives for further research and development and ensure that European taxpayers’ money was “spent sensibly...READ MORE
- Using ‘Ancient History’: FDA Says Study Will Offer Plasma Therapy For COVID-19 (newsmax.com)The convalescent sera option for containing COVID-19 (jci.org)
The Food and Drug Administration on Friday announced a national study led by the Mayo Clinic that will help hospitals offer an experimental plasma therapy for COVID-19 patients, and track how they fare...The therapeutic agents—convalescent plasma and hyperimmune globulin—are both derived from the blood of people who have recovered from the disease...What the history books call “convalescent serum” was most famously used during the 1918 flu pandemic, and also against measles, bacterial pneumonia and numerous other infections before modern medicine came along...Some hospitals are already administering convalescent plasma to critical COVID-19 patients, a so-called “compassionate use” that in this case is allowed by what the FDA calls an emergency Investigational New Drug authorization...READ MORE
- U.S. FDA says malaria drugs in shortage as coronavirus drives up demand (reuters.com)Current and Resolved Drug Shortages and Discontinuations Reported to FDA (accessdata.fda.gov)
The U.S. Food and Drug Administration said malaria drugs hydroxychloroquine and chloroquine are in shortage due to a surge in demand because of the coronavirus pandemic...The drugs, which have been tried with some success to treat the illness caused by the virus, were added to the agency's website that lists drug shortages on Tuesday...Studies are underway in a number of countries to see whether hydroxychloroquine and the related malaria drug chloroquine may be effective in controlling the spread of coronavirus, which has led to a surge in demand for the treatments...READ MORE
- UK ramps up coronavirus trials but results ‘a few months away’ (reuters.com)
Britain said...it was launching the biggest clinical trial of possible treatments for coronavirus in the world but a leading health official cautioned that the results were likely a few months away...Almost 1,000 patients from 132 hospitals had been recruited in 15 days and thousands more were expected to join in the coming weeks...The trial is testing medicines more commonly used to treat malaria and HIV, and is designed so that when further medicines are identified, they can be added to the study within days...England’s Deputy Chief Medical Officer Jonathan Van-Tam said the next round of clinical trials should include new medicines, including those that might be in development for other diseases and might “have a role to play”...READ MORE
- Pharmaceutical Companies Lend Support to Hydroxychloroquine Clinical Trials for COVID-19 (pharmacytimes.com)
Pharmaceutical companies Novartis and Rising Pharmaceuticals are taking action to support the latest clinical trials exploring hydroxychloroquine as a treatment for the coronavirus disease 2019...Novartis announced March 30 that it is donating 20,000 doses of hydroxychloroquine to the University of Washington for a COVID-19 PEP clinical trial, which is expected to provide approximately 2000 patients with a 14 post-exposure regimen...Earlier in March, Novartis committed to donating up to 130 million doses, or 200mg tablets, of generic hydroxychloroquine to support COVID-19 research...Rising Pharmaceuticals has announced a collaborative agreement with the Division of Infectious Disease and International Medicine at the University of Minnesota, Department of Infectious Disease on a clinical trial investigating hydroxychloroquine as a preventive treatment for COVID-19...READ MORE
- Coronavirus (COVID-19) Update: FDA Continues to Accelerate Development of Novel Therapies for COVID-19 (fda.gov)
As part of the Trump Administration’s all-hands-on-deck approach across public, academic and private sectors to combat the COVID-19 pandemic, the U.S. Food and Drug Administration stood up a new program to expedite the development of potentially safe and effective life-saving treatments. The program, known as the Coronavirus Treatment Acceleration Program (CTAP), is using every tool at the agency’s disposal to bring new therapies to sick patients as quickly as possible, while at the same time supporting research to further evaluate whether these medical countermeasures are safe and effective for treating patients infected with this novel virus.,,READ MORE