- FDA Identifies “Essential Medicines” for US (pharmtech.com)
As part of the administration’s campaign to reduce the United States’ reliance on an increasingly global pharmaceutical supply chain and to minimize potential shortages for critical drugs, FDA has published a list of those drugs and medical products considered critical for addressing public health emergencies. The list will provide a basis for bolstering biopharmaceutical production at home of essential medicines and medical products and for addressing supply chain vulnerabilities...READ MORE
- Three Western states join California in screening any FDA-approved coronavirus vaccine (sfchronicle.com)
Washington, Oregon and Nevada will join California to independently review any coronavirus vaccine before distributing it to the public...Gov. Gavin Newsom said Tuesday that the three states would identify their own public health experts to participate in the scientific review committee he announced last week, which was charged with ensuring that any vaccine approved by the U.S. Food and Drug Administration is safe and effective...California has also formed a second committee to develop guidelines for the ethical distribution of vaccines, Newsom said, addressing questions about who should receive the first doses and how to allocate potentially limited supplies...READ MORE
- Gilead wins first FDA approval for COVID-19 treatment, Veklury (bioworld.com)
Following a rapid course of development and testing, Gilead Sciences Inc. has secured the first and only FDA approval for a COVID-19 treatment, the antiviral Veklury (remdesivir). Previously authorized only for emergency use (EUA) in the U.S., the drug can now be prescribed to adults and children 12 and older weighing at least 40 kg for the treatment of COVID-19 requiring hospitalization...READ MORE
- California says it will independently review coronavirus vaccine (reuters.com)
A California panel of experts will independently review the safety of new coronavirus vaccines and initial plans for distribution, Governor Gavin Newsom said...The 11-person panel specializing in topics such as epidemiology, biostatistics, and infectious disease will review any vaccine approved by the Food and Drug Administration before it is distributed to state residents, Newsom told a news conference...The U.S. government’s efforts to speed development of a COVID-19 vaccine - and promises by U.S. President Donald Trump that one could be available prior to the Nov. 3 presidential election - has led to concerns of political interference in the regulatory process at the expense of safety. The FDA has vowed to ensure the safety of COVID-19 vaccines before approving them...READ MORE
- Sanofi, SK flu shots halted in Singapore as South Korea post-vaccination deaths climb to 59 (fiercepharma.com)
Deaths after flu vaccination keep rising in South Korea. But as local health authorities work to calm concerned citizens by refuting a connection between the two, a fellow Asian country has taken the precautionary measure of suspending two shots given to people who later died...Singapore has temporarily pulled its backing for SK Bioscience’s SKYCellflu Quadrivalent and Sanofi Pasteur’s VaxigripTetra, the Ministry of Health said...READ MORE
- CMS: Medicare to cover COVID-19 vaccine at no cost to beneficiaries (fiercehealthcare.com)
The Trump administration announced it will cover a vaccine for COVID-19 at no cost to Medicare beneficiaries...The Centers for Medicare & Medicaid Services released an interim rule on Wednesday that aims to remove regulatory barriers for seniors to get the vaccine, which is expected to be approved by early next year...READ MORE
- CVS pushing for pharmacy technicians to be able to administer COVID-19 vaccines (fiercehealthcare.com)CVS Health to hire 15,000 across the U.S. in fourth quarter (cvshealth.com)CVS to add 1K rapid-result COVID-19 test sites at pharmacies (fiercehealthcare.com)
CVS Health is pushing for pharmacy technicians to be allowed to administer COVID-19 vaccines...The healthcare giant is hiring more than 10,000 full- and part-time pharmacy technicians in Q4 in anticipation of flu season, and urging for them to have an expanded scope of practice that would allow them to vaccinate patients for the novel coronavirus under supervision from an immunization-certified pharmacist...CVS said that allowing technicians to administer vaccines would "help fill the urgent need to safely and quickly scale distribution of a vaccine and extend the capacity of the health care workforce to address the pandemic."...READ MORE
- Takeda, Moderna partner up to bring 50M coronavirus shots to Japan (fiercepharma.com)
Japanese drugmaker Takeda has quickly emerged as one of the leading forces in the fight against COVID-19 as a vaccine manufacturing partner and R&D lead on a convalescent plasma-based therapy. Now, Takeda will help another biotech distribute its potential COVID-19 vaccine in the drugmaker's home country...Moderna has tapped Takeda to bring 50 million doses of its mRNA-based COVID-19 shots to Japanese shores as part of a government-backed vaccine distribution effort...Takeda will import and distribute Moderna's shot, dubbed mRNA-1273, starting in the first half of next year, as well as handle local regulatory approvals...READ MORE
- FDA won’t require manufacturing inspections for emergency COVID-19 vaccine use: Bloomberg (fiercepharma.com)
As the leading COVID-19 vaccines move closer to potential emergency use, the research and regulatory processes have been intensely scrutinized. But there’s one area that hasn’t seen as much attention so far—manufacturing facility inspections...COVID-19 vaccine developers seeking FDA emergency use authorizations won’t have to undergo pre-approval site inspections...The FDA conducts thousands of facility inspections per year, and some find flaws that drug or vaccine companies must remedy before launching new products...READ MORE
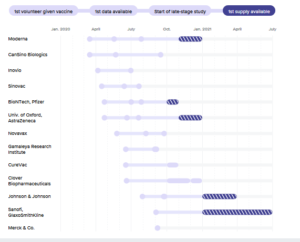
- The coronavirus vaccine frontrunners are advancing quickly. Here’s where they stand. (biopharmadive.com)
Fast progress has spurred hopes that several vaccines could succeed, spurring jockeying among governments to secure supplies...Scientists, drugmakers and governments are moving with unprecedented haste to develop a vaccine against the new coronavirus...The fastest of them have already delivered promising data from initial human studies, and further results from larger tests should come quickly over the next three to six months...The goal...is to have a vaccine ready for use in some fashion by the end of the year, or early next. Doing so would be a scientific feat with few parallels. No vaccine has ever been developed so quickly, never mind manufactured for the world...READ MORE