- Gilead Sciences sends additional doses of COVID-19 therapy remdesivir to EU amid shortages (fiercepharma.com)
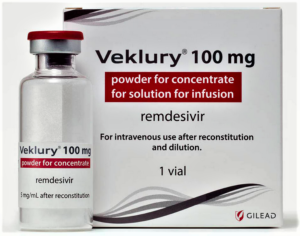
As one of few COVID-19 therapeutics with global regulators on board, Gilead Sciences' remdesivir saw immediate U.S. demand that has somewhat tapered off in recent months. But in the EU, some nations have seen spot shortages, and Gilead is stepping in with more supply...Gilead has shipped enough doses of its antiviral Veklury (remdesivir) to the EU to treat 3,400 patients—or 20,300 doses—after countries reported rationing and shortages of the COVID-19 therapy, a Gilead spokeswoman confirmed Wednesday. That $8.2 million order would be enough to meet roughly one to two weeks of treatment supply...READ MOVE
- Deregulate Pharmacists Now To Increase COVID-19 Vaccine Uptake (reason.com)
Enabling tens of millions of Americans to get themselves speedily vaccinated against COVID-19 will be a huge logistics challenge. A new policy brief from the Mercatus Center, a think tank at George Mason University, argues that we could greatly accelerate the process by removing the complex state regulations that prevent pharmacists from administering vaccines...The...report recommends that state regulators relax age restrictions on pharmacist-administered vaccinations; issue statewide standing orders authorizing pharmacists to administer vaccines without requiring a physician-written prescription for each patient; and revise regulations, as Oregon has, to permit pharmacists to administer all of the vaccines recommended by the CDC's Advisory Committee on Immunization Practices. The latter recommendation "prevents lag in vaccine administration due to boards or legislatures having to approve individually named vaccines for pharmacist administration."...READ MORE
- 3 Coronavirus Vaccines in Phase 3 Clinical Studies: How They Stack Up Against Each Other (fool.com)
Any way you look at it, the numbers related to coronavirus vaccine candidates in development are impressive. In less than nine months, researchers have advanced 180 experimental coronavirus vaccines at least into preclinical testing. Thirty-five of those candidates are now in clinical studies...The field narrows considerably, though, when we look only at vaccine candidates that have made it to late-stage testing. Currently, only three experimental coronavirus vaccines are in phase 3 clinical trials being conducted in the U.S.:
-AstraZeneca
-Pfizer
-ModernaHere's how these three coronavirus vaccines in phase 3 testing stack up against each other...READ MORE
- CDC Head and Trump Spar Over COVID-19 Vaccine Timeline (biospace.com)
Robert Redfield, director of the U.S. Centers for Disease Control and Prevention, testified...before the U.S. Senate...He noted that although a vaccine against COVID-19 will likely be available and to begin dosing in November or December of this year, it will be limited. Getting the entire U.S. population vaccinated will likely take “six to nine months.”...He also said that face masks are “the most important, powerful public health tool we have.” He added, “I might even go so far as to say that this face mask is more guaranteed to protect me against COVID than when I take a COVID vaccine.”...READ MORE
- Gilead Sciences takes back remdesivir distribution as demand drops (fiercepharma.com)
After a somewhat chaotic initial rollout by the U.S. government, Gilead Sciences is now taking distribution of COVID-19 drug remdesivir into its own hands...Starting Thursday, Gilead will directly sell remdesivir, branded as Veklury, to American hospitals, ending a five-month phase when the U.S. Department of Health and Human services was responsible for allocating it...AmerisourceBergen will remain the sole U.S. distributor through the end of the year...READ MORE
- Trump says may block stricter FDA guidelines for COVID-19 vaccine (reuters.com)
U.S. President Donald Trump said...he may or may not approve any new, more stringent FDA standards for an emergency authorization of a COVID-19 vaccine, saying such a proposal would appear political...The Washington Post reported on Tuesday the U.S. Food and Drug Administration would issue the guidance to boost transparency and public trust as health experts have become increasingly concerned the Trump administration might be interfering in the approval process to rush out a vaccine...READ MORE
- Tech startups developing rapid COVID-19 tests to be taken completely at home (fiercehealthcare.com)
Computer vision company Gauss teamed up with biotechnology company Cellex to develop a rapid, at-home and point-of-care COVID-19 antigen test...While there are other at-home COVID-19 diagnostic tests available, at least two companies are working on rapid antigen tests that can be performed by people at home without involving a laboratory...The Gauss and Cellex test has not yet gained Food and Drug Administration approval. The companies are pushing for FDA Emergency Use Authorization some time this fall...READ MORE
- Physicians Start Remdesivir Therapy for Trump (newsmax.com)Trump says he’s leaving Walter Reed Medical Center tonight (reviewjournal.com)
Medical specialists have elected to start Remdesivir therapy for President Donald Trump...Trump has completed his first dose of the drug and is resting comfortably...He is not requiring any supplemental oxygen...Later in the day he was also transported to Walter Reed military hospital for a few days' of treatment as a precautionary measure, though officials said he is expected to remain on the job...READ MORE
- BREAKING: FDA will make it HARDER to get a coronavirus vaccine approved in a move that could block Trump’s plan to have a shot before Election Day (dailymail.co.uk)U.S. FDA to tighten coronavirus vaccine authorization standards ahead of election - paper (reuters.com)
The US Food and Drug Administration... is expected to issue new, tougher requirements for its approval of a coronavirus vaccine, a move that could obliterate the chances of a shot getting emergency use authorization before Election Day...Regulators could publish the new approval standards as early as this week, and will do so publicly in an effort to bolster Americans' eroded trust in the US to ensure the safety a COVID-19 vaccine...President Trump and his Operation Warp Speed initiative have been pushing for months to have a coronavirus vaccine approved ahead of the November 3...READ MORE
- CVS aims to have 4,000 drive-thru testing sites open by mid-October (fiercehealthcare.com)
CVS Health is planning to double the number of its drive-thru testing sites by mid-October, the healthcare giant announced...CVS intends to add more than 2,000 sites at its pharmacies in the next several weeks, bringing its total to more than 4,000 nationwide. The new locations will be opened in waves, beginning with 400 new sites opening on Friday...READ MORE