- A vaccine alone won’t stop Covid-19. We also need a trusted plan for it (statnews.com)
Safe and effective vaccines represent the most effective way to restore the health and economic security disrupted by the Covid-19 pandemic. To help achieve that goal, the U.S. government launched Operation Warp Speed...to accelerate development and manufacturing of several Covid-19 vaccines, with a goal of having 300 million doses available to the U.S. population by January 2021...Operation Warp Speed is expediting vaccine development primarily by moving clinical trials forward without pauses between phases, and by scaling up manufacturing capacity before knowing if a candidate works...Covid-19 vaccines can help stop the pandemic only if people trust them and want to be vaccinated. To earn and keep the trust of the American people, our government needs to ensure three key needs are met before launching any immunization campaign...READ MORE
- Ensure transparency and confidence in FDA decisions
- Ensure robust active safety monitoring as Covid-19 vaccines are rolled out
- Ensure the distribution and administration of Covid-19 vaccines are equitable and well-executed
- AstraZeneca Covid-19 vaccine study put on hold due to suspected adverse reaction in participant in the U.K. (statnews.com)
A large, Phase 3 study testing a Covid-19 vaccine being developed by AstraZeneca and the University of Oxford at dozens of sites across the U.S. has been put on hold due to a suspected serious adverse reaction in a participant in the United Kingdom...A spokesperson for AstraZeneca, a front-runner in the race for a Covid-19 vaccine, said in a statement that the company’s “standard review process triggered a pause to vaccination to allow review of safety data.”...READ MORE
- Costa Rica researchers to trial coronavirus treatment from horse antibodies (reuters.com)Costa Rica researchers to trial coronavirus treatment from horse antibodies (scientificamerican.com)
Researchers in Costa Rica are due to begin trials of an inexpensive coronavirus treatment based on antibodies taken from horses injected with the SARS-Cov-2, the virus that causes COVID-19...Developed by University of Costa Rica’s Clodomiro Picado Institute, the equine antibodies medication is to be tested on 26 patients from mid-September...Costa Rican authorities hope to be able to begin applying the treatment more widely in hospitals if the results from the phase 2 study are encouraging. There are 471 hospitalized coronavirus patients in Costa Rica...Similar efforts are also underway in Argentina and Brazil, while scientists in Belgium are using llamas...READ MORE
- In China’s Xinjiang, forced medication accompanies lockdown (apnews.com)
The government in China’s far northwest Xinjiang region is resorting to draconian measures to combat the coronavirus, including physically locking residents in homes, imposing quarantines of more than 40 days and arresting those who do not comply. Furthermore, in what experts call a breach of medical ethics, some residents are being coerced into swallowing traditional Chinese medicine, according to government notices, social media posts and interviews with three people in quarantine in Xinjiang. There is a lack of rigorous clinical data showing traditional Chinese medicine works against the virus, and one of the herbal remedies used in Xinjiang, Qingfei Paidu, includes ingredients banned in Germany, Switzerland, the U.S. and other countries for high levels of toxins and carcinogens...READ MORE
- Generic Drug Shortage Solutions on the Horizon (drugtopics.com)
Shortages of vital generic drugs—particularly since the start of the coronavirus disease 2019 pandemic in the US—are well known by pharmacy teams in both health systems and community settings...although pharmacy associations appreciate the Trump administration’s efforts to move more pharmaceutical and active pharmaceutical ingredient manufacturing to the United States...they say government initiatives must include an overall plan for drug pricing, payment model, and supply chain transparency...READ MORE
- NIH panel says data doesn’t support plasma use for COVID-19 (biopharmadive.com)
A panel of advisers for the National Institutes of Health was not convinced convalescent plasma should be used to treat COVID-19, a recommendation that appears to conflict with a controversial decision by the Food and Drug Administration last week to issue an emergency authorization for the blood-derived treatment...The panel...reviewed the same data cited by the FDA, but concluded it to be "insufficient" to recommend "either for or against the use of convalescent plasma for the treatment of COVID-19."...the same panel cautioned against using hydroxychloroquine in treating coronavirus disease three weeks after the FDA cleared emergency use of the malaria pill...READ MORE
- New CDC report shows 94% of COVID-19 deaths in US had contributing conditions (fox8.com)Excess Deaths Associated with COVID-19 (cdc.gov)
The Centers for Disease Control and Prevention released new data last week that depicts how many Americans who have died from COVID-19 also had other contributing conditions...According to the report, only 6% of deaths have COVID-19 as the only cause mentioned, revealing that 94% of patients who died from coronavirus also had other “health conditions and contributing causes.”...The CDC explains that their data uses provisional death counts to “deliver the most complete and accurate picture of lives lost to COVID-19.”...These numbers are based on death certificates, which the organization says are the most reliable source of data. Death certificates reportedly contain information that is not available anywhere else and includes comorbid conditions, race and ethnicity, and place of death...The CDC says provisional death counts may not match counts from other sources, such as numbers from county health departments, because death certificates take time to be completed, states report at different rates, it takes officials extra time to code COVID-19 deaths, and because other reporting systems use different definitions or methods for counting deaths...The organization adds that provisional data is not yet complete, provisional counts are not final and are subject to change, and that death counts should not be compared across states...READ MORE
- Researchers identify nanobody that may prevent COVID-19 infection (phys.org)An alpaca nanobody neutralizes SARS-CoV-2 byblocking receptor interaction (nature.com)
Researchers at Karolinska Institutet in Sweden have identified a small neutralizing antibody, a so-called nanobody, that has the capacity to block SARS-CoV-2 from entering human cells. The researchers believe this nanobody has the potential to be developed as an antiviral treatment against COVID-19...READ MORE
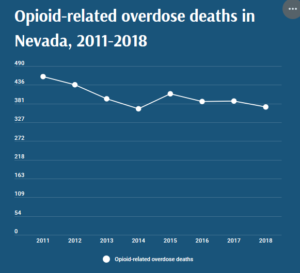
- With opioid-related overdoses on the rise, health care providers try preparing everyday Nevadans to respond to a crisis (thenevadaindependent.com)
With opioid-related overdoses on the rise, health care providers try preparing everyday Nevadans to respond to a crisis..From January to May 2020, Nevada saw 23 percent more opioid-related overdose deaths than during the same period in 2019, and similar trends are being seen across the country...Opioid-related overdose deaths peaked in Nevada in 2011 and have been on the decline since then, but around the U.S., rates have been rising throughout the COVID-19 pandemic. The American Medical Association released a report in mid-August citing news reports from 40 states and Washington, D.C. showing a rise in overdoses and illicit substance abuse since March...According to data from the Nevada Overdose Data to Action Program, there have been 197 opioid-involved drug overdose deaths in 2020 as of May 31, a 23 percent increase over the 160 counted in the first five months of 2019. April and May had the highest rates of overdose-related emergency room visits, with a 25 percent increase over the three months prior...READ MORE
- Dozens of U.S. hospitals poised to defy FDA’s directive on COVID-19 plasma (fiercepharma.com)
Dozens of major hospitals across the U.S. are grappling with whether to ignore a federal decision allowing broader emergency use of blood plasma from recovered COVID-19 patients to treat the disease in favor of dedicating their resources to a gold-standard clinical trial that could help settle the science for good...As many as 45 hospitals from coast to coast have expressed interest in collaborating on a randomized, controlled clinical trial...Officials at some hospitals said they are considering committing only to the clinical trial—and either avoiding or minimizing use of convalescent plasma through an emergency use authorization issued...by the federal Food and Drug Administration...The response comes amid concerns that the Trump administration pressured the FDA into approving broader use of convalescent plasma...A National Institutes of Health panel this week countered the FDA’s decision, saying that the therapy “should not be considered the standard of care for the treatment of patients with COVID-19” and that well-designed trials are needed to determine whether the therapy is helpful. Data so far suggests the treatment could be beneficial, but it’s not definitive...READ MORE