- With vaccines in more stores, pharmacies go on hiring spree (pharmacist.com)
Dozens of pharmacy chains and grocery stores with pharmacy counters are slated to start offering COVID-19 vaccinations this week, creating a wealth of employment opportunities. The companies are vying with one another—offering lucrative signing bonuses, in some cases—to hire established pharmacists, pharmacy technicians, student pharmacists, nurses, and other help. CVS Health is working toward 15,000 new vaccine-related hires, Walgreens has targeted 9,000, Kroger plans to recruit nearly 1,000 health care workers, and Rite Aid is looking to fill more than 2,000 pharmacy jobs...READ MORE
- Nevada climbs out of bottom in administering vaccine, CDC says (reviewjournal.com)
Nevada no longer has one of the worst COVID-19 vaccination rates per capita in the U.S., according to federal data...The Silver State had consistently ranked among the bottom five states at administering vaccine for weeks. It now ranks 12th worst, the Centers for Disease Control and Prevention reported...READ MORE
- AstraZeneca, Oxford race to update COVID-19 vaccine as study flags weak action against variant (fiercepharma.com)
It didn’t take long before a morale boost for AstraZeneca’s COVID-19 vaccine was overshadowed by disappointment over its waned protection against a newly emerged coronavirus variant...A new study has found AZ’s COVID-19 shot offered “minimal protection” against mild to moderate disease caused by the B.1.351 variant, which was first identified in South Africa, the University of Oxford, the original developer of the vaccine, said...READ MORE
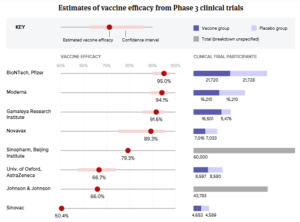
- While Pfizer and Moderna hold the lead, new data from J&J, Novavax show there’s ‘room for everyone’ in the market: analysts (fiercepharma.com)
With Novavax and Johnson & Johnson data now in hand, the world has efficacy numbers on at least five COVID-19 vaccine programs. That's plenty for analysts to parse—and they're busy doing not only that, but also sizing up the market as the field of top players grows...The upshot? Blockbuster sales to go around, but Pfizer and Moderna will continue to lead the pack, partly thanks to their ability to quickly deploy mRNA against new variants. And for Moderna, at least, that means almost $14 billion in projected sales just this year from one team of analysts...READ MORE
- Pfizer to nearly halve COVID-19 vaccine production timeline, sterile injectables VP says (fiercepharma.com)
DNA production—the first step in Pfizer's vaccine manufacturing process—could soon take just nine to 10 days, rather than 16...With an upsized production goal of 2 billion COVID-19 vaccine doses this year, Pfizer and its German partner BioNTech aren’t resting on their laurels now that their shot, Comirnaty, has emergency nods in the U.S., Europe and beyond. As the companies continue to build out capacity, manufacturing efficiency is getting its own boost...The time it takes the company to produce a COVID-19 vaccine batch could soon be cut from 110 days to an average of just 60...READ MORE
- The first coronavirus vaccines have arrived. Here’s where the rest stand. (biopharmadive.com)
Study results showed vaccines from J&J; and Novavax to be effective against COVID-19. But seemingly weaker protection versus new virus variants have raised concerns...Scientists, drugmakers and governments have moved with unprecedented haste to develop a vaccine against the new coronavirus...The fastest of them have completed studies proving their vaccines can protect against COVID-19. A half dozen shots from developers in the U.S., U.K., Germany, China and Russia have now been cleared by regulators for emergency use...READ MORE
- Pfizer, Johnson & Johnson balk at shareholders’ push for COVID-19 vaccine pricing info (fiercepharma.com)
What's the rationale behind COVID-19 vaccine and drug prices? You don’t have a need to know—or so say a couple of the leading contenders...Two major players in the pandemic fight, Pfizer and Johnson & Johnson, are urging the Securities and Exchange Commission to forestall shareholder resolutions that would require them to disclose how they set prices on their COVID-19 vaccines...Several not-for-profit groups are pushing the two companies—along with fellow pharmas Eli Lilly, Gilead, Merck & Co. and Regeneron—for information on their drug and vaccine pricing decisions, citing the federal money all have received, either for supplies, R&D or manufacturing scale-up. Or all three...READ MORE
- Nearly 60% of Critical Care Pharmacists Report Burnout (pharmacypracticenews.com)
With many ICUs reaching or exceeding maximum capacity in spring 2020 due to the COVID-19 pandemic, it may not be a surprise that about 60% of critical care pharmacists reported feeling burned out in a recent national survey...In an electronic survey that queried critical care pharmacists about their institutions and number of activities performed in May and June 2020, 128 of 221 respondents (58%) reported feelings of burnout such as emotional exhaustion and depersonalization...READ MORE
- FDA clears Lilly’s COVID-19 antibody cocktail for emergency use (biopharmadive.com)
The Food and Drug Administration...cleared an antibody drug cocktail from Eli Lilly for emergency use for treating people recently diagnosed with COVID-19...The cocktail, a combination of two coronavirus-targeting antibodies, is authorized only for people with mild or moderate symptoms of COVID-19, but who are at high risk of the disease's worst effects due to age, underlying medical conditions or other preexisting conditions...The combination pairs Lilly's bamlanivimab...with another antibody called etesevimab that the drugmaker developed in partnership with China's Junshi Biosciences. Each antibody targets a separate section of the "spike" protein used by the SARS-CoV-2 virus to breach the body's cells — a feature designed to preserve the drug's effectiveness even as the virus mutates...READ MORE
- No need ‘to start at square one’: FDA plans to lay out a speedy path for COVID-19 vaccines, drugs against variants (fiercepharma.com)
New coronavirus variants have prompted COVID-19 vaccine makers to start developing updates to their existing offerings. To speed their journey to a pandemic-fatigued public, the FDA says it’s developing expedited review rules for the follow-up shots...The FDA’s working on guidance for the types of data needed to support changes to COVID-19 vaccines. The new rules would provide for “streamlined clinical programs” that can demonstrate an immune response to new variants and “can be executed quickly,” FDA’s acting commissioner, Janet Woodcock, said in a statement...READ MORE