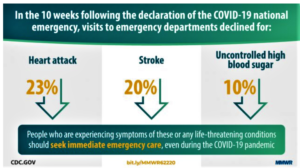
- U.S. emergency visits due to heart attacks fell during early days of COVID-19 (reuters.com)Potential Indirect Effects of the COVID-19 Pandemic on Use of Emergency Departments for Acute Life-Threatening Conditions — United States, January–May 2020 (cdc.gov)
Fewer Americans were admitted to emergency departments with life-threatening conditions such as heart attacks during the initial months of the COVID-19 pandemic,..The study suggests that patients may be delaying or avoiding seeking care because of fear of COVID-19, researchers from the U.S. Centers for Disease Control and Prevention said...study...showed that the number of deaths in New York City from causes other than COVID-19 rose by more than 5,000 people above the seasonal norm during the first two months of the pandemic...Visits to the emergency department because of heart attacks fell 23%, ten weeks after the pandemic was declared a national emergency, compared with ten weeks before the emergency declaration...READ MORE
- OHSU’s COVID-19 Study Accused Of Racial Bias (opb.org)
Charges of racial bias in the design of an Oregon study of COVID-19’s spread are raising questions about whether it will do anything to help Black and Latino communities, which have been among those hardest hit by the pandemic...“All it will be able to say is if white people are fine. And then we open up counties and people of color will die,” said Andres Lopez, research director for the Coalition of Communities of Color, a Portland-based alliance of organizations representing a number of different communities of color...The Key to Oregon Study, which plans to enlist 100,000 Oregonians and monitor them for a year for COVID-19 symptoms, will include what its designers are calling “a focus on enrolling people who fully represent the state, including our diversity in geography, socioeconomic status and communities of color.”...critics doubt Key to Oregon will succeed in its goal. They say the study design is fundamentally flawed, and that those flaws could have been avoided if people of color had been brought to the table when the study was being created...READ MORE
-Responding with listening sessions
-Advocates say study design suppresses Black and Latino voices
-OHSU methodology overlooks lessons of the past
-The principle of ‘nothing about us without us’
-OHSU researchers respond - Show me the data: U.S. doctors skeptical of reported COVID breakthrough (reuters.com)
The report on...a powerful treatment for the new coronavirus brought skepticism along with optimism among U.S. doctors, who said the recent withdrawal of an influential COVID-19 study left them wanting to see more data...Global pressure to find a cure or vaccine has accelerated the process of reporting coronavirus study results, feeding confusion over whether therapies have been proven effective. One influential COVID study was withdrawn this month by respected British medical journal The Lancet over data concerns...Researchers in Britain said dexamethasone, used to fight inflammation in other diseases, reduced death rates of the most severely ill COVID-19 patients by around a third, and they would work to publish full details as soon as possible...But hours later South Korea’s top health official cautioned about the use of the drug for COVID-19 patients due to potential side effects...READ MORE
- NHS waiting list will more than double to 10 million by Christmas, health experts warn (standard.co.uk)
Around 10 million people will be on the waiting list for NHS treatment by the end of the year, more than double the current figure, health bosses have warned...Projections show the combined effects of keeping up social distancing, the backlog of treatments and challenges around staffing mean the list could rise from 4.2 million currently to around 10 million by Christmas...This is the most realistic scenario, and assumes the health service making a steady return to full capacity within the next 12 months...The pessimistic scenario, according to the NHS Confederation, assumes a second wave of Covid-19 and a lack of treatments or a vaccine, pushing the waiting list to around 11 million by the end of the year...The confederation, which represents health and care leaders, published a new report warning that the health service in England "faces an uphill battle" as it continues to manage thousands of sick and recovering Covid-19 patients while also trying to restart services such as those for cancer, stroke and heart disease...READ MORE
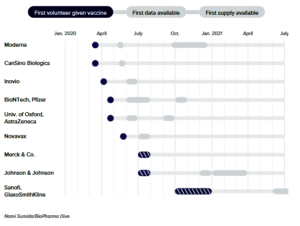
- The coronavirus vaccine frontrunners have emerged. Here’s where they stand
Fast progress by several companies has spurred hopes that a vaccine is coming soon, spurring jockeying among governments to secure supplies…Scientists, drugmakers and governments are moving with unprecedented speed to deliver a vaccine to protect against the new coronavirus…The fastest of them have already delivered preliminary data from human studies, and further results from others should come quickly as the year progresses…The goal, at least in the U.S., is to have a vaccine ready for use in some fashion by the end of the year, or early next. Doing so would be a scientific feat with few parallels. No vaccine has ever been developed so quickly, never mind manufactured for the world…Vaccine frontrunners plan for fast development…READ MORE
- FDA Releases Drug Interaction Warning for Remdesivir and Hydroxychloroquine (drugtopics.com)
The FDA released a warning to health care providers concerning an update on potential drug reactions for remdesivir, an antiviral drug that is being evaluated as a potential treatment for the novel coronavirus disease 2019 and has also been granted emergency use authorization status for treating hospitalized patients with severe COVID-19...The FDA is also revising their fact sheet for health care providers to include the warning that co-administration of remdesivir and chloroquine phosphate or hydroxychloroquine sulfate may result in less effective antiviral activity of remdesivir. The revised fact sheet also clarifies dosing and administration recommendations, and will provide additional safety data and updates from clinical trials from the National Institutes of Health and Gilead Sciences, Inc, the company that sponsors the drug and has donated 607,000 vials of remdesivir to the United States government...READ MORE
- EU calls for global alliance to buy COVID-19 vaccines up front (reuters.com)
The European Commission called...for global leaders to cooperate to buy bulk quantities of potential COVID-19 vaccines, to avoid “harmful competition” in the race for a shot and ensure any future vaccine is available for poor countries...With around a dozen potential vaccines now in human trials, rich countries have been rushing to buy up doses in advance from pharmaceutical companies, to make sure they will have enough supply should any prove successful...The European Commission, the EU executive arm, is worried that such competition could raise the prices of vaccines for everyone, and also leave many countries, mostly poor ones, struggling to obtain a supply...READ MORE
- Pharmacy Groups Praise New York COVID-19 Pharmacist Vaccination Law (drugtopics.com)
New York pharmacists can provide the coronavirus disease 2019 vaccine when it becomes available, according to new legislation...The Community Pharmacy Association of New York State and NACDS praised the new law, based on legislation (S. 8182-A / A. 10508-A) that adds COVID-19 to the list of illnesses for which pharmacists can vaccinate...The legislation was...was signed into law by New York Governor Andrew Cuomo...“Now is the time to make sure that people will be able to get their COVID-19 vaccination as soon as it becomes available. It is essential that all states continue to remove barriers for pharmacies to help meet the needs of patients during this phase of the pandemic,”...READ MORE
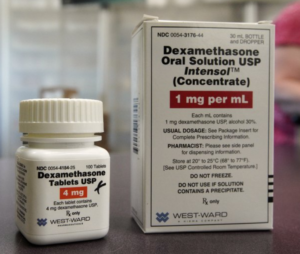
- Cheap drug is first shown to improve COVID-19 survival (apnews.com)
Researchers in England say they have the first evidence that a drug can improve COVID-19 survival: A cheap, widely available steroid reduced deaths by up to one third in severely ill hospitalized patients...The results were announced Tuesday and the British government immediately authorized the drug’s use across the United Kingdom for coronavirus patients like those who did well in the study. Researchers said they would publish results soon in a medical journal, and several independent experts said it’s important to see details to know how much of a difference the drug, dexamethasone, might make and for whom...READ MORE
- UK begins coronavirus vaccine trial; France pledges funding (apnews.com)
Scientists at Imperial College London will start immunizing people in Britain this week with their experimental coronavirus shot, while pharmaceutical company Sanofi and the French government announced more than 800 million euros ($890 million) in investment...as part of the worldwide race to find an effective vaccine...About a dozen vaccine candidates are currently in early stages of testing in thousands of people. There are no guarantees any will work but there’s increasing hope that at least some could be ready by the end of the year...the British government said 300 healthy people will be immunized with two doses of the COVID-19 vaccine candidate developed at Imperial, which has been backed by 41 million pounds ($51 million) in government funding...READ MORE