- Hospitals are starting to get their coronavirus cash infusion (axios.com)
The federal government is sending $64 billion to hospitals, post-acute facilities and other medical providers to help cope with the coronavirus fallout...Even though more funding is coming, safety net and rural hospitals fear they are getting a raw deal from the way some of the money is being distributed...Hospitals and other providers requested funding to offset higher labor and supply costs as well as lost revenue from elective surgeries and procedures that had to be halted. Those federal funds, part of the most recent stimulus package, are now flowing..."Rural hospitals are going to close this year," Alan Morgan, CEO of the National Rural Health Association, said of the initial funding distribution. "There will be a lot of blame to go around."...READ MORE
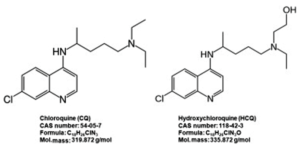
- Special Report: Doctors embrace drug touted by Trump for COVID-19, without hard evidence it works (reuters.com)Doctors Express Hope, Questions About Using Malaria Drugs To Combat Coronavirus (thefederalist.com)Scoop: Inside the epic White House fight over hydroxychloroquine (axios.com)
The decades-old drug that President Donald Trump has persistently promoted as a potential weapon against COVID-19 has within a matter of weeks become a standard of care in areas of the United States hit hard by the pandemic — though doctors prescribing it have no idea whether it works...Doctors and pharmacists from more than half a dozen large healthcare systems in New York, Louisiana, Massachusetts, Ohio, Washington and California told Reuters they are routinely using hydroxychloroquine on patients hospitalized with COVID-19. At the same time, several said they have seen no evidence that the drug, used for years to treat malaria and autoimmune disorders, has any effect on the virus...READ MORE
- Greece suggests EU buy patent rights for vaccines and coronavirus tests: FAZ (reuters.com)
Greece has suggested EU member states jointly buy patent rights for vaccines against COVID-19 and rapid tests under development to help ensure that if they are effective they are quickly distributed to those in need across the bloc...Greek Prime Minister Kyriakos Mitsotakis said finding a solution for a rapid distribution of vaccines, when they are available, is difficult but also urgent...At least 20 vaccines against COVID-19 are under development, many of which are subsidised by individual governments or charities...“Ideally, once their efficacy has been proven, such vaccines should be distributed as quickly and fairly as possible, and at a reasonable cost,”...Purchasing such patent rights would give global pharmaceutical companies incentives for further research and development and ensure that European taxpayers’ money was “spent sensibly...READ MORE
- Using ‘Ancient History’: FDA Says Study Will Offer Plasma Therapy For COVID-19 (newsmax.com)The convalescent sera option for containing COVID-19 (jci.org)
The Food and Drug Administration on Friday announced a national study led by the Mayo Clinic that will help hospitals offer an experimental plasma therapy for COVID-19 patients, and track how they fare...The therapeutic agents—convalescent plasma and hyperimmune globulin—are both derived from the blood of people who have recovered from the disease...What the history books call “convalescent serum” was most famously used during the 1918 flu pandemic, and also against measles, bacterial pneumonia and numerous other infections before modern medicine came along...Some hospitals are already administering convalescent plasma to critical COVID-19 patients, a so-called “compassionate use” that in this case is allowed by what the FDA calls an emergency Investigational New Drug authorization...READ MORE
- State working to fix Nevada’s coronavirus test supply shortage (reviewjournal.com)
Nevada’s northern and southern labs are out of coronavirus testing swabs, but officials expect to get more kits this week, including rapid-result test kits, Gov. Steve Sisolak said Monday...At a coronavirus briefing, Sisolak said the state has received 4,000 test swabs from the federal government and 3,000 reagent liquid kits that are used to test the samples...Sisolak said that while federal officials provided test components, “we did not get complete kits.”...State health workers continue to struggle to find enough kits to fill growing patient demands, and some clinics have shut down temporarily in Las Vegas as they wait for new supplies...READ MORE
- CVS to launch two new drive-through COVID-19 testing sites (reuters.com)
CVS Health Corp said that it will launch two new drive-through COVID-19 testing sites in Georgia and Rhode Island...using new, faster tests than had previously been available, with up to four more locations to follow...The company said both drive-through testing sites will use testing equipment made by Abbott Laboratories that can deliver results within minutes. It expects to be able to perform around 1,000 tests per day at each site...“We want to get some experience under our belt with these sites and understand exactly sort of what the volume looks like. And we’ll also be improving the logistics associated with each of the sites over time,” CVS Chief Medical Officer Troy Brennan said...READ MORE
- UK ramps up coronavirus trials but results ‘a few months away’ (reuters.com)
Britain said...it was launching the biggest clinical trial of possible treatments for coronavirus in the world but a leading health official cautioned that the results were likely a few months away...Almost 1,000 patients from 132 hospitals had been recruited in 15 days and thousands more were expected to join in the coming weeks...The trial is testing medicines more commonly used to treat malaria and HIV, and is designed so that when further medicines are identified, they can be added to the study within days...England’s Deputy Chief Medical Officer Jonathan Van-Tam said the next round of clinical trials should include new medicines, including those that might be in development for other diseases and might “have a role to play”...READ MORE
- FDA’s Hahn: No sign China has affected U.S. drug supply during coronavirus pandemic (fiercepharma.com)
With pharmaceutical supply chains under immense pressure due to the novel coronavirus, China's role as a global ingredient producer has come under scrutiny. Despite fears the East Asian nation could shut off the tap for U.S. drugs, the FDA said it hasn't yet noticed major signs for concern...The FDA hasn't seen a shortage of active pharmaceutical ingredients (APIs) sourced from China due to the ongoing novel coronavirus outbreak but is "closely monitoring the situation," FDA Commissioner Stephen Hahn told Fox News...Hahn highlighted reported spot shortages of certain drugs due to increased demand but said China's API tap is still running, despite escalating rhetoric between the Chinese and U.S. governments. Hahn highlighted the Trump administration's push to develop "advanced" manufacturing stateside to help drive greater redundancy in the supply chain...READ MORE
- Federal government approves Nevada’s request for major disaster declaration, allowing access to additional resources (thenevadaindependent.com)Sisolak: More PPE, tests needed to combat COVID-19, state may need stricter enforcement of social distancing (thenevadaindependent.com)
Nevada’s request for a major disaster declaration, opening up additional paths for federal assistance under the national emergency proclamation, has been approved, Gov. Steve Sisolak announced Saturday afternoon...The declaration, which was requested by the state on Tuesday, will allow federal dollars to flow to Nevada to support Carson City and local governments’ ongoing responses to the COVID-19 pandemic. Federal funding is expected to be made available to governmental agencies and certain private nonprofit organizations for emergency protective measures...READ MORE
- Unlike FDA, European regulators refuse to clear chloroquine for COVID-19 without data (fiercepharma.com)
...days after the FDA gave them (chloroquine and hydroxychloroquine) an emergency approval to treat COVID-19...European regulators are limiting their COVID-19 use to clinical trials only...The decision comes as limited data—some of it questionable—rolls in about the drugs and their potential as COVID-19 therapies. A French study that's made headlines continues to draw fire, but brand-new data from China add to the positive case...In guidance...the European Medicines Agency restricted general use of the drugs—already approved to treat malaria and autoimmune diseases—to patients taking them for approved indications. COVID-19 patients can receive the drugs as part of clinical trials or through national emergency use programs...READ MORE