- Pharma’s reputation rehab: A whopping two-thirds of Americans now offer a thumbs-up, Harris Poll finds (fiercepharma.com)
Almost two-thirds of Americans now give the pharma industry a thumbs up. It’s a stunning reversal from just one year ago when only about one-third (32%) rated the industry positively, according to The Harris Poll surveys...In its most recent February poll, 62% rated the pharma industry as a 5, 6 or 7 on a 7-point scale, with 1 equating to “very bad” and 7 to “very good.” That’s an increase of 30 percentage points since January 2020, before the pandemic hit U.S. shores...And that's a key point: The hockey-stick upturn stems directly from pharma's proactive response to COVID-19...READ MORE
- With vaccines in more stores, pharmacies go on hiring spree (pharmacist.com)
Dozens of pharmacy chains and grocery stores with pharmacy counters are slated to start offering COVID-19 vaccinations this week, creating a wealth of employment opportunities. The companies are vying with one another—offering lucrative signing bonuses, in some cases—to hire established pharmacists, pharmacy technicians, student pharmacists, nurses, and other help. CVS Health is working toward 15,000 new vaccine-related hires, Walgreens has targeted 9,000, Kroger plans to recruit nearly 1,000 health care workers, and Rite Aid is looking to fill more than 2,000 pharmacy jobs...READ MORE
- Nevada climbs out of bottom in administering vaccine, CDC says (reviewjournal.com)
Nevada no longer has one of the worst COVID-19 vaccination rates per capita in the U.S., according to federal data...The Silver State had consistently ranked among the bottom five states at administering vaccine for weeks. It now ranks 12th worst, the Centers for Disease Control and Prevention reported...READ MORE
- Pfizer seeks to store vaccine at higher temperatures, easing logistics (reuters.com)
Pfizer Inc and BioNTech SE have asked the U.S. health regulator to relax requirements for their COVID-19 vaccine to be stored at ultra-low temperatures, potentially allowing it to be kept in pharmacy freezers...Approval by the Food and Drug Administration could send a strong signal to other regulators around the world that may ease distribution of the shot in lower-income countries...The companies have submitted new temperature data to the FDA to support an update to the current label that would allow vials to be stored at -25 to -15 degrees Celsius (-13°F to 5°F) for a total of two weeks...READ MORE
- Pfizer to nearly halve COVID-19 vaccine production timeline, sterile injectables VP says (fiercepharma.com)
DNA production—the first step in Pfizer's vaccine manufacturing process—could soon take just nine to 10 days, rather than 16...With an upsized production goal of 2 billion COVID-19 vaccine doses this year, Pfizer and its German partner BioNTech aren’t resting on their laurels now that their shot, Comirnaty, has emergency nods in the U.S., Europe and beyond. As the companies continue to build out capacity, manufacturing efficiency is getting its own boost...The time it takes the company to produce a COVID-19 vaccine batch could soon be cut from 110 days to an average of just 60...READ MORE
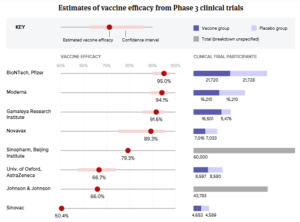
- The first coronavirus vaccines have arrived. Here’s where the rest stand. (biopharmadive.com)
Study results showed vaccines from J&J; and Novavax to be effective against COVID-19. But seemingly weaker protection versus new virus variants have raised concerns...Scientists, drugmakers and governments have moved with unprecedented haste to develop a vaccine against the new coronavirus...The fastest of them have completed studies proving their vaccines can protect against COVID-19. A half dozen shots from developers in the U.S., U.K., Germany, China and Russia have now been cleared by regulators for emergency use...READ MORE
- WHO authorizes AstraZeneca’s COVID vaccine for emergency use (kold.com)
The World Health Organization has granted an emergency authorization to AstraZeneca’s coronavirus vaccine, a move that should allow the U.N. agency’s partners to ship millions of doses to countries worldwide as part of a U.N.-backed program to tame the pandemic...the WHO said it was clearing the AstraZeneca vaccines made by the Serum Institute of India and South Korea’s AstraZeneca-SKBio...“Countries with no access to vaccines to date will finally be able to start vaccinating their health workers and populations at risk,” said Dr Mariângela Simão, the WHO’s Assistant-Director General for Access to Medicines and Health Products....READ MORE
- Nearly 60% of Critical Care Pharmacists Report Burnout (pharmacypracticenews.com)
With many ICUs reaching or exceeding maximum capacity in spring 2020 due to the COVID-19 pandemic, it may not be a surprise that about 60% of critical care pharmacists reported feeling burned out in a recent national survey...In an electronic survey that queried critical care pharmacists about their institutions and number of activities performed in May and June 2020, 128 of 221 respondents (58%) reported feelings of burnout such as emotional exhaustion and depersonalization...READ MORE
- FDA clears Lilly’s COVID-19 antibody cocktail for emergency use (biopharmadive.com)
The Food and Drug Administration...cleared an antibody drug cocktail from Eli Lilly for emergency use for treating people recently diagnosed with COVID-19...The cocktail, a combination of two coronavirus-targeting antibodies, is authorized only for people with mild or moderate symptoms of COVID-19, but who are at high risk of the disease's worst effects due to age, underlying medical conditions or other preexisting conditions...The combination pairs Lilly's bamlanivimab...with another antibody called etesevimab that the drugmaker developed in partnership with China's Junshi Biosciences. Each antibody targets a separate section of the "spike" protein used by the SARS-CoV-2 virus to breach the body's cells — a feature designed to preserve the drug's effectiveness even as the virus mutates...READ MORE