- FDA User Fees to Rise and Fall as New Fee Agreements Move Forward (pharmtech.com)
Biopharmaceutical companies will pay more than $3 million to file an application seeking FDA approval of a new drug application or biologics license application during fiscal year 2022. This record charge to evaluate new drugs and biologics reflects agency analysis of additional personnel and resources needed to process its growing workload. The annual program fee paid by each manufacturer with marketed prescription drugs holds fairly even at about $370,000. And while the actual increase in the application fee is not that much, passing the $3 million mark has drawn attention and further concern about FDA’s overdependence on revenue from regulated industry...READ MORE
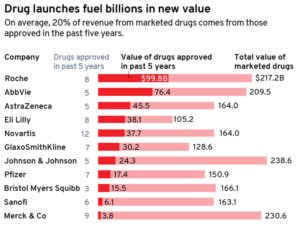
- Who’s getting the most out of their R&D engine? Pharma’s top 11, ranked (fiercepharma.com)
Drugmakers have myriad tools in their arsenal when looking to grow sales. They can acquire marketed drugs, raise prices or focus on growing the reach of their existing medicines. But it's often new drug approvals that reign supreme and ultimately prove the worth of a company's development engine...covered in a recent Evaluate Vantage report, the team at Fierce Pharma took a close look at the recent approvals for 11 of the world's biggest drugmakers by revenue. Specifically, we're highlighting the dollar value of the industry's launches from the last five years and analyzing how the new meds fit into each company's overall portfolio...READ MORE
- Most Americans Oppose Biden’s Prescription Drug Price Controls (realclearhealth.com)
In response to U.S. prescription drug spending rapidly outpacing inflation, reaching an estimated $358.7 billion in 2020, President Biden last week demanded that Congress adopt strict federal price controls on prescription drugs...to cap annual out-of-pocket drug spending for Medicare beneficiaries, empowering the Secretary of Health and Human Services to choose pharmaceutical winners and losers, and financially crippling penalties for pharmaceutical companies that don’t acquiesce to price controls — are likely to have disastrous effects on the availability of critical medications. What’s more, they’re deeply unpopular with the overwhelming majority of Americans, and could prove to be a significant fiscal and health liability for our nation in the years to come...READ MORE
- Federal appeals court sides with HHS in spat over Medicare Advantage overpayment rule (fiercehealthcare.com)
UnitedHealthcare has lost an appeal in a case over Medicare Advantage overpayments...The health insurance giant initially won in lower federal court, but the Department of Health and Human Services filed an appeal in spring 2020. The ruling is the latest in the legal back-and-forth over a 2014 proposed rule that could change how much money MA plans have to pay the feds for diagnostic errors...READ MORE
- CMS delays enforcement of key parts of price transparency rule by 6 months (fiercehealthcare.com)
The Biden administration has delayed enforcement of key parts of a major insurer price transparency rule by six months until July 1, 2022, to give plans more time to comply...The Centers for Medicare & Medicaid Services announced the change in a new guidance released Friday focusing on the final price transparency rule released last October under the Trump administration. The guidance focuses on a requirement that certain health plans disclose online their in-network provider rates for covered items and services, out-of-network allowed amounts and billed charges for certain items and services...READ MORE
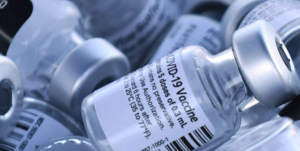
- Pfizer, BioNTech score FDA’s first full COVID-19 vaccine nod, quickly triggering stricter mandates (fiercepharma.com)
The U.S. FDA has awarded the first full approval for a COVID-19 vaccine to Pfizer and its German partner BioNTech, a historic decision that comes weeks ahead of its previously expected Labor Day deadline...Pfizer’s jab, now approved for people aged 16 and older, will remain under an emergency nod for adolescents aged 12 to 15...The agency’s full approval for Pfizer’s mRNA shot, now marketed as Comirnaty, is expected to spark a wave of vaccine mandates from companies, universities and organizations awaiting the agency’s final sign-off...READ MORE
- Is NICE becoming nicer? England’s cost-effectiveness watchdog lays out plans to speed access to new medicines (fiercepharma.com)
England's cost-effectiveness watchdog, typically a fierce critic of pharma's launch prices, says it’s revamping the way it reviews new drugs, devices, diagnostics and more in a push to provide greater access, sooner...The National Institute for Health and Care Excellence, or NICE, rolled out its planned remodel...aimed at providing “faster, fairer” access to the drugs and devices it reviews for National Health Services (NHS) patients...READ MORE
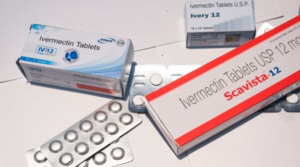
- FDA Says “Stop It” to Self-Medicating for COVID-19 with Unapproved Treatments (biospace.com)
“You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This is the Food and Drug Administration's recently tweeted advice to Americans seeking out alternative, unapproved treatments for COVID-19...The comment is aimed mainly at Mississippi residents as a follow-up to the state’s official Health Network Alert issued Friday. The Magnolia State has experienced increasing calls to its poison control line, with 70% of the calls related to the ingestion of the livestock formulation of anti-parasitic drug ivermectin...While ivermectin is FDA-approved to treat and prevent parasite infections, the drug commonly found at most local feed stores is highly concentrated for large animals such as horses and cows. In that type of formulation, it can be highly toxic to humans...READ MORE
- Expanding Access Takes Telepharmacy to the Next Level (drugtopics.com)
During the COVID-19 pandemic many hospitals and health care services teams turned to telepharmacy to reduce delays in providing medications to patients while social distancing practices were in place. Hospitals quickly found that telepharmacy benefitted pharmacy services by providing patients with more efficient access to medical care and reducing costs. Thus, hospitals were able to focus more on patient care and less on logistics, which led to higher turnaround times and increased patient satisfaction...READ MORE
- FDA set to issue full approval for Pfizer vaccine on Monday (msn.com)
The New York Times reports that the Pfizer shot will be the first of the coronavirus jabs to be cleared by the FDA...According to the report, the FDA originally had planned to approve the vaccine before Labor Day, but decided to accelerate its ruling...The full approval of the shot also paves the way for employers and private companies to mandate employees and patrons to be vaccinated...READ MORE