- Telehealth providers cheer DEA move to temporarily extend virtual prescribing flexibilities (fiercehealthcare.com)
The clock is ticking on the end of the COVID-19 public health emergency and, with it, telehealth flexibilities that enabled doctors to virtually prescribe controlled medications to their patients...Facing major backlash to its proposed rules released in February, the Drug Enforcement Administration is looking to buy some time to reconsider whether it should enforce stricter limits around the prescribing of controlled substances via telehealth...The Biden administration said at the time that the new rule seeks to provide safeguards to prevent online over-prescribing of controlled medications. Teleprescribing has been touted as a robust tool for bringing medications for opioid use disorder to rural areas in the ongoing treatment of the opioid epidemic...READ MORE
- Supreme Court maintains access to abortion pill, blocking restrictions on its use (biopharmadive.com)
The stay suspends an order by a Texas judge that had invalidated the FDA’s approval of mifepristone, keeping it available while a circuit court hears the case...The Supreme Court on Friday allowed the abortion pill mifepristone to remain on the market for the time being, suspending a lower court order that would have curtailed its availability in the U.S....The 7-2 ruling stays a verdict earlier this month by U.S. District Court Judge Matthew Kacsmaryk invalidating the Food and Drug Administration’s 2000 approval of mifepristone. Justices Samuel Alito and Clarence Thomas dissented...READ MORE
- As US drug shortages persist, House committee presses FDA for answers (fiercepharma.com)
With drug shortages becoming increasingly common...House Republicans are pressing the FDA for answers. At the heart of the investigation is whether the FDA has done enough to prevent and respond to the current spate of shortfalls, which have affected cancer meds and nonprescription painkillers alike...In a letter...Republican leaders from the House Committee on Energy and Commerce pressed FDA Commissioner...to respond 10 questions on the FDA’s tracking of scarce medicines, its inspection priorities and what the agency has done to parse and prevent shortages...READ MORE
- Teladoc-owned BetterHelp to pay $7.8M to online therapy users for alleged data misuse, per FTC order (fiercehealthcare.com)
The Federal Trade Commission has reached a settlement with online therapy company BetterHelp...over allegations that it shared consumers’ health data with companies like Facebook and Snapchat for advertising purposes...BetterHelp is banned from sharing consumers’ health data, including sensitive information about mental health challenges, with third parties for marketing and ad targeting...BetterHelp also agreed to pay $7.8 million to consumers to settle charges that it revealed consumers’ sensitive data with third parties for advertising after promising to keep such data private...READ MORE
- One killed, 4 injured in Atlanta medical office mass shooting (fiercehealthcare.com)
Four people were injured and one woman was killed in a mass shooting Wednesday in a downtown Atlanta medical office waiting room, local law enforcement said...The suspected gunman, a 24-year-old man, entered Midtown medical building and shot the victims around noon, police said. He escaped in a stolen vehicle, kicking off a manhunt before he was taken into custody without incident later that evening, they said...The victim who was killed has been identified as Amy St. Pierre, a 39-year-old who worked for the Centers for Disease Control and Prevention, according to a statement from the agency...READ MORE
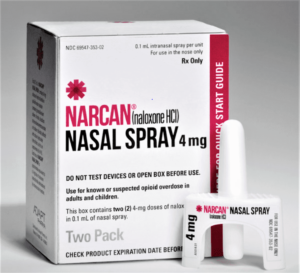
- Emergent makes history with first FDA nod for over-the-counter naloxone (fiercepharma.com)
...the FDA approved the first over-the-counter naloxone product...the U.S.’ drug regulator blessed Emergent’s 4mg Narcan nasal spray with a nonprescription nod, teeing up direct-to-consumer sales at places like drug stores, convenience stores, supermarkets and gas stations...the timeline on which the product will be made available at stores—as well as its price—is up to Emergent...Other formulations and dosages of naloxone will remain prescription only, though there are access laws that technically permit pharmacies across all 50 states to dispense the emergency drug without a doctor’s note...READ MORE
- PBM Formulary Exclusions List Reaches All-Time High (drugtopics.com)
The number of drugs excluded from the three largest pharmacy benefit manager formularies reached an all-time high in 2023, despite concerns that profits are being put ahead of patient access. This year, CVS Caremark, Optum Rx and Express Scripts — which together handle 80% of all prescriptions in the United States — each have roughly 600 medications on their standard formulary exclusion lists...Exclusions leave patients with fewer options for treatment unless they can afford the out-of-pocket costs of buying drugs that are not covered by the insurer. What is even more disturbing are the trends within the trend of formulary exclusions, critics say,,,READ MORE
- Biotech layoffs gather pace as industry downturn persists (biopharmadive.com)
More than 5,000 employees have been let go from biotech and pharmaceutical companies so far this year...At least five dozen biotechnology companies have laid off employees so far this year in a sector-wide contraction that has reached large and small drugmakers alike...Brought on by enduring funding challenges, the consolidation has resulted in roughly 3,200 biotech employees losing their jobs between Jan. 1 and early April...While the workforce reductions aren’t a new development — more than 100 biotech companies conducted layoffs last year — the pace of announcements has accelerated, suggesting the industry hasn’t recovered from 2022’s market downturn...READ MORE
- Bipartisan caucus in US Congress looks to boost domestic drug manufacturing (fiercepharma.com)
...the Domestic Pharmaceutical Manufacturing Caucus for the 118th Congress...will focus on domestic production of finished drugs and active pharmaceutical ingredients...Members of the caucus said they will look to advance legislation that incentivizes more domestic production for essential medicines as part of an effort to reduce U.S. reliance on foreign countries. The lawmakers also aim to head off potential supply chain disruptions and ensure a steady supply of pharmaceuticals in the event of public health emergencies...READ MORE
- Eli Lilly slashed insulin prices. This starts a race to the bottom (fiercehealthcare.com)
When drugmaker Eli Lilly announced...it will slash the list price for some of its insulin products following years of criticism from lawmakers and activists that the price of the lifesaving hormone had become unaffordable, the news raised questions about what will happen to other efforts to provide low-cost insulin...Civica...has said it plans to begin selling biosimilar insulin for roughly $30 per vial by 2024—$5 more than the new price of Eli Lilly’s generic insulin...Mark Cuban said his new company, the Mark Cuban Cost Plus Drug Co., planned to sell low-cost insulin. And California is poised to launch an ambitious program to manufacture its own brand of the hormone, as well as generics of other high-priced prescription drugs...READ MORE