- US: WHO not sharing enough info about China virus probe (apnews.com)
A senior U.S. government official complained...that the World Health Organization has not shared enough information about its planned mission to China to investigate the animal origins of the coronavirus...Garrett Grisby of the U.S. Department of Health and Human Services griped that the criteria for WHO’s China mission had not been shared with other nations. He spoke during a weeklong meeting of the U.H. health agency’s member countries...“The (terms of reference) were not negotiated in a transparent way with all WHO member states,” he said via video conference, referring to the mission’s criteria. “Understanding the origins of COVID-19 through a transparent and inclusive investigation is what must be done.”...READ MORE
- Pfizer’s Early Data Shows Vaccine Is More Than 90% Effective (nytimes.com)After Pfizer's vaccine data, analysts say COVID19 drugmakers face questions about long-term sales prospects (fiercepharma.com)Pfizer/BioNTech Candidate COVID-19 Vaccine Report More Than 90% Efficacy, But Details Sparse (pharmacypracticenews.com)
Pfizer announced positive early results from its coronavirus vaccine trial, cementing the lead in a frenzied global race that has unfolded at record-breaking speed...Pfizer, which developed the vaccine with the German drugmaker BioNTech, released only sparse details from its clinical trial, based on the first formal review of the data by an outside panel of experts...The company said that the analysis found that the vaccine was more than 90 percent effective in preventing the disease among trial volunteers who had no evidence of prior coronavirus infection. If the results hold up, that level of protection would put it on par with highly effective childhood vaccines for diseases such as measles. No serious safety concerns have been observed, the company said...READ MORE
- EXCLUSIVE: Surveillance video shows dozens of looters trashing Oakland pharmacy for hours; where were police? (abc7news.com)
Police in Oakland and other East Bay cities have been dealing with a rash of brazen robberies and other incidents since Tuesday night...Among those businesses hit, was Oakland's longtime High Street Pharmacy...It started with a blow torch at the back door of the 73-year-old pharmacy, as seen on the store's surveillance cameras. Several men show up just after 10:30 p.m., cut their way through a metal back door and then the marathon begins...For five hours, until after 3:30 in the morning, dozens of people run into the pharmacy, smashing computers, wrecking displays, stealing drugs and other merchandise and ransacking a business that..."We feel like we deserve a little bit better," said owner Gareen Avakian, who told us she's heard from neighbors who called 911 almost as soon as the brazen break-in began. An alarm company also apparently tried to call, but Oakland police didn't respond for five hours...READ MORE
- Aetna, city of New Haven hit with OCR fines after data breach (healthcareitnews.com)
The U.S. Department of Health and Human Services' Office for Civil Rights leveraged $1,000,000 in fines against Aetna Life Insurance Company and $202,400 against the city of New Haven, Connecticut, to settle potential HIPAA violations...The fines are just the latest moves on the part of OCR to enforce HIPAA regulations around protected health information..."When individuals contract for health insurance, they expect plans to keep their medical information safe from public exposure. Unfortunately, Aetna's failure to follow the HIPAA Rules resulted in three breaches in a six-month period, leading to this million dollar settlement," said OCR Director Roger Severino in a statement...READ MORE
- Brazil health regulator suspends Chinese-made vaccine trials (apnews.com)
Brazil’s health regulator halted clinical trials of the potential coronavirus vaccine CoronaVac, citing an “adverse, serious event,” according to a statement it posted to its website Monday night...The potential vaccine is being developed by Chinese biopharmaceutical firm Sinovac and in Brazil would be mostly produced by Sao Paulo’s state-run Butantan Institute. Butantan said in a statement that it was surprised by Anvisa’s decision and that it would hold a news conference Tuesday...The CoronaVac shot has stirred controversy in Brazil, where President Jair Bolsonaro has cast doubt on its prospective effectiveness. He sparked confusion last month when he publicly rejected it, saying Brazilians would not be used as guinea pigs. The declaration followed news that his health minister, Eduardo Pazuello, had agreed to purchase CoronaVac doses produced locally by Butantan...READ MORE
- Coronavirus (COVID-19) Update: FDA Authorizes First Test that Detects Neutralizing Antibodies from Recent or Prior SARS-CoV-2 Infection (fda.gov)SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) Kit (RUO) (genscript.com)
...the U.S. Food and Drug Administration authorized the first serology test that detects neutralizing antibodies from recent or prior SARS-CoV-2 infection, which are antibodies that bind to a specific part of a pathogen and have been observed in a laboratory setting to decrease SARS-CoV-2 viral infection of cells. The FDA issued an emergency use authorization for the cPass SARS-CoV-2 Neutralization Antibody Detection Kit, which specifically detects this type of antibody...Although the FDA has previously issued EUAs to more than 50 antibody tests, those tests only detect the presence of binding antibodies. Binding antibodies bind to a pathogen, such as a virus, but do not necessarily decrease the infection and destruction of cells...READ MORE
- Oregon leads the way in decriminalizing hard drugs (apnews.com)Monumental Night for Drug Policy Reform (drugpolicy.org)
In a first in the nation, Oregon has rejected charging drug users with criminal offenses, with voters passing a ballot measure that decriminalizes possession of heroin, methamphetamine, LSD, oxycodone and other hard drugs...“Today’s victory is a landmark declaration that the time has come to stop criminalizing people for drug use,” said Kassandra Frederique, executive director of the Drug Policy Alliance, which was behind the measure. “Measure 110 is arguably the biggest blow to the war on drugs to date.”...The measure completely changes how Oregon’s justice system treats those who are found with personal-use amounts of the hard drugs...READ MORE
- Fierce Pharma Politics—Biden puts COVID-19 transition task force on the job (fiercepharma.com)
Two days after news organizations called the U.S. presidential election for Joe Biden, the President-elect is laying out his COVID-19 advisory board, tapping experts in the fields of infectious diseases, regulatory matters and more...Co-chairing the coronavirus transition task force will be former Surgeon General Vivek Murthy, former FDA commissioner David Kessler and Yale University associate professor of medicine and epidemiology Marcella Nunez-Smith. Alongside them, a long list of health experts—including ousted BARDA director Rick Bright and Michael Osterholm, the director of the University of Minnesota’s Center for Infectious Disease Research and Policy—will serve on the team...READ MORE
- GSK pledges renewed environmental commitment with aggressive climate change and nature targets (fiercepharma.com)
GlaxoSmithKline is committing to a new environmental plan with two ambitions: eliminate its impact on climate change and neutralize its impact on nature by 2030...In environmental parlance, the goal is a net zero impact on climate change and a net positive impact on nature by the end of the decade...The twofold approach means GSK will reduce its environmental footprint as much as possible—and where it can’t reduce, implement restoration programs—while also contributing more to nature than it takes out...READ MORE
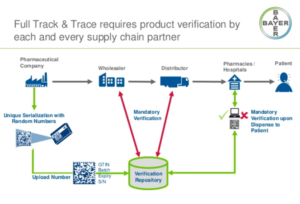
- Pharmacies must comply with certain track-and-trace requirements by November 27, FDA says (pharmacist.com)
Despite APhA’s concerns that compliance with certain Drug Supply Chain Security Act requirements undermines pandemic response efforts, FDA has confirmed that “dispensers,” including pharmacies, must meet a requirement that goes into effect on November 27, 2020. APhA and pharmacy partners had requested that FDA delay enforcement of certain portions of the DSCSA—also known as “track and trace”—mandate...READ MORE