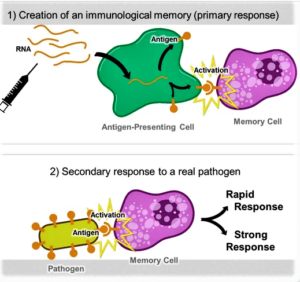
- Moderna won’t enforce COVID-19 vaccine patents during pandemic (fiercepharma.com)What Are mRNA Vaccines, and Could They Work Against COVID-19? (smithsonianmag.com)
Moderna, a biotech advancing one of the leading COVID-19 vaccine candidates, has faced questions for several months over its patents and intellectual property. Responding to investor questions, the company now says it won't enforce its vaccine patents against other companies during the pandemic...company President Stephen Hoge said Moderna is “quite studiously not asserting infringement.” Without naming names, the biotech says other COVID-19 vaccines in development might be using Moderna-patented technology...“We’re not interested in using that IP to decrease the number of vaccines available in a pandemic,”...Further, the company is open to licensing its technology after the pandemic. Moderna made the pledge in response to investor questions around its patents...READ MORE
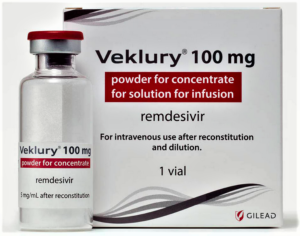
- Gilead Sciences sends additional doses of COVID-19 therapy remdesivir to EU amid shortages (fiercepharma.com)
As one of few COVID-19 therapeutics with global regulators on board, Gilead Sciences' remdesivir saw immediate U.S. demand that has somewhat tapered off in recent months. But in the EU, some nations have seen spot shortages, and Gilead is stepping in with more supply...Gilead has shipped enough doses of its antiviral Veklury (remdesivir) to the EU to treat 3,400 patients—or 20,300 doses—after countries reported rationing and shortages of the COVID-19 therapy, a Gilead spokeswoman confirmed Wednesday. That $8.2 million order would be enough to meet roughly one to two weeks of treatment supply...READ MOVE
- Access to medical oxygen: a glaring global inequity (statnews.com)
Since the World Health Organization declared Covid-19 a global pandemic more than six months ago, we have passed many grim milestones...One of the glaring global inequities creating enormous quality-of-care challenges for doctors, nurses, and other health care providers, especially in countries with fragile health systems, is limited access to medical oxygen. In low-resource settings around the world, where many lack access even to electricity and clean water, oxygen availability is severely limited...The disparity in safe, reliable access to medical oxygen — which is needed to treat a range of diseases and health conditions, and is absolutely essential for treating Covid-19 — is further compounded as health systems are overwhelmed by surges of patients...READ MORE
- Physicians Start Remdesivir Therapy for Trump (newsmax.com)Trump says he’s leaving Walter Reed Medical Center tonight (reviewjournal.com)
Medical specialists have elected to start Remdesivir therapy for President Donald Trump...Trump has completed his first dose of the drug and is resting comfortably...He is not requiring any supplemental oxygen...Later in the day he was also transported to Walter Reed military hospital for a few days' of treatment as a precautionary measure, though officials said he is expected to remain on the job...READ MORE
- Verma doubles down on supporting Medicaid work requirements as enrollment swells (fiercehealthcare.com)
The head of Centers for Medicare & Medicaid Services reiterated support for Medicaid work requirements as enrollment in the program swells this year...CMS Administrator Seema Verma spoke about work requirements...Her remarks come less than a month after a study in Health Affairs found that work requirements in Arkansas did not lead to more employment...“I am supportive of states making decisions about their programs and deciding what has worked best,” Verma said...She added that 20 states have been interested in the work requirements program...CMS has approved waivers for 10 states but currently no states are running a work requirement program. Arkansas, Indiana, Michigan and New Hampshire all started work requirement programs, but court rulings struck them down. Utah implemented its work requirement program in January, but it was suspended in April due to COVID-19...Verma said that the goal of the programs were to help improve the health of able-bodied Medicaid beneficiaries...READ MORE
- Early-stage nano-carrier uses miRNA to deploy cancer meds on contact with diseased cells (fiercepharma.com)
Cancer cells could play a key role in the delivery of tumor-fighting drugs, thanks to a new DNA transport system that keeps its therapeutic payload locked away until it comes into contact with diseased cells...Scientists at the Technical University of Munich and the KTH Royal Institute of Technology...say they’ve developed a synthetic DNA transport system for targeted delivery of cancer drugs...the team has created a nano-carrier using mucin, glycerol and synthetic DNA, which wraps up cancer drugs in a secure package that can only be unlocked by a certain RNA sequence unique to diseased cells...READ MORE
- AI Identifies Sex-Specific Adverse Events With High Precision (drugtopics.com)
Researchers have developed an algorithm that is able to accurately predict sex differences in drug response through the implementation of pharmacogenomic data...The study...presented AwareDX, a resource that uses machine learning to understand sex-specific adverse drug effects with the potential for use in drug discovery, repositioning, and pharmacogenetic studies, as well as for further analyses of electronic health records and clinical trials, according to investigators...Women are twice as likely as men to develop adverse drug reactions to a drug, partly due to differences in pharmacokinetics and pharmacodynamics that induce increased drug bioavailability and sensitivity to medication and women [remain] severely underrepresented in clinical trials...READ MORE
- Regeneron antibodies in demand after Trump treatment, doctors seek more data (reuters.com)
Patients are asking to join clinical trials of antibody-based COVID-19 drugs after U.S. President Donald Trump was treated last week with an experimental therapy from Regeneron Pharmaceuticals Inc...Medical experts said more data is needed to assess the treatment’s efficacy before wider use should be allowed...The company said...that it has submitted a request to the U.S. Food and Drug Administration for an emergency use authorization for its antibody combination...READ MORE
- Cutting off H-1B visas will hurt the biopharma industry (statnews.com)
...Presidential Proclamation 10051 suspended immigration into the United States for anyone holding H-1B, J-1, and L visas, and suspended granting new ones. Most worrisome for the pharmaceutical industry is the ban on H-1B visas, because it will limit, and in some instances entirely prevent, biopharma companies from recruiting the specialized talent they need...the suspension and limitation are aimed at ensuring “that the presence in the United States of H-1B nonimmigrants does not disadvantage United States workers.” The proclamation ends on Dec. 31, at which point the limitations will cease or the administration may extend them as it sees fit...READ MORE
- Gilead Sciences takes back remdesivir distribution as demand drops (fiercepharma.com)
After a somewhat chaotic initial rollout by the U.S. government, Gilead Sciences is now taking distribution of COVID-19 drug remdesivir into its own hands...Starting Thursday, Gilead will directly sell remdesivir, branded as Veklury, to American hospitals, ending a five-month phase when the U.S. Department of Health and Human services was responsible for allocating it...AmerisourceBergen will remain the sole U.S. distributor through the end of the year...READ MORE