- Why a data security sting lurks in COVID-19’s long tail (healthcareitnews.com)
Hospital executive minds have understandably been distracted since the start of 2020, but the impact of the emergence of SarsCoV2 has not been limited to its physical toll. It has also torn into data security defenses and exposed patient privacy...The word ‘unprecedented’ seems to have been used on a daily basis during the COVID-19 pandemic, particularly when it comes to the impact of the virus on patients, clinicians, resources and care delivery. But it has resonated equally strongly with hospital chief information security officers, with its power to either stiffen resolve or ratchet up already stretched nervous tension as data security faces a whole new scale and level of cyber threats...READ MORE
- Following court ruling, NIH warns drug and device companies to post missing trial data (statnews.com)
Hundreds of drug companies, medical device manufacturers, and universities owe the public a decade’s worth of missing data from clinical trials, federal officials warned last week...New rules issued last week in the wake of a federal court ruling in February instructed clinical trial sponsors to submit missing data for trials conducted between 2007 and 2017 “as soon as possible.” For years, many trials conducted during that span have largely been exempted from reporting their data to ClinicalTrials.gov, a public database, meaning a decade of data about approved drugs and medical devices has never been made public...The court’s ruling, and the federal government’s decision not to appeal it and instead to urge trial sponsors to submit the missing information, represent a major win for transparency advocates, who for years have fought to recover the decadelong gap in publicly available clinical trial data...READ MORE
- Telemedicine is booming — but many people still face huge barriers to virtual care (statnews.com)Telehealth seems here to stay – so how can it be improved? (healthcareitnews.com)
As Covid-19 drives many patients away from in-person care and toward virtual visits, experts warn that the nation’s most vulnerable members may be shut out of the booming telehealth business...Federal policymakers temporarily relaxed regulations to make it easier to provide virtual care during the pandemic, fueling a shift toward telemedicine that has become so popular among patients and providers that there are now a number of proposals to make the changes permanent. Just this week, President Trump signed an executive order that would permanently extend some of those policies...But a pair of new studies published this week show that there are barriers to virtual visits that regulatory changes alone can’t fix...READ MORE
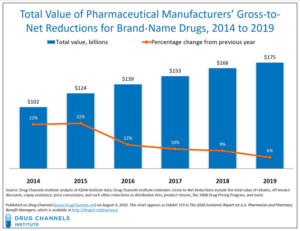
- The Gross-to-Net Bubble Hit $175 Billion in 2019: Why Patients Need Rebate Reform (drugchannels.net)
Last Friday’s Executive Orders revived the government’s effort to reform rebates in federal programs. Whether that effort succeeds, today's update reminds us what's still at stake in reforming rebates within the U.S. drug channel...For 2019, Drug Channels Institute estimates that the gross-to-net bubble—the dollar gap between sales at brand-name drugs' list prices and their sales at net prices after rebates and other reductions—reached $175 billion...The bubble reflects—and drives—many of patients’ problems and misunderstandings of U.S. drug prices...However, the political and practical challenges to rebate reform remain daunting. Few people grasp the complex economic interplay of patient out-of-pocket spending, cost-shifting, premiums, and payer incentives...READ MORE
- Rules on prescription drug prices in Georgia tightened in Kemp-signed bill (gwinnettdailypost.com)
Gov. Brian Kemp signed legislation...tightening rules on third-party companies that play a role in negotiating pharmaceutical drug prices between insurers and local pharmacies in Georgia...The bill...requires...pharmacy benefits managers to set drug prices within a national average, a move aimed at reining in excessively high prescription prices...Senate Bill 313...also forces PBMs to offer up full rebates to health plans that are typically given by drugmakers, rather than pocketing a portion...And PBMs will need to submit to new audits by the state Department of Community Health as well as requirements for publishing data on prescription prices online...READ MORE
- House Dems launch probe into Kodak’s unexpected $765M drug manufacturing loan from the feds (fiercepharma.com)
The Trump administration’s surprising $765 million deal that would enable former photography giant Kodak to start making drug ingredients has yet to be finalized, and it’s already attracted plenty of controversy. First, insider trading allegations from the U.S. Senate—and now, an investigation by House Democrats...The lawmakers are questioning why the government picked Kodak, which has little experience in pharma manufacturing, for such a major pact, as well as suspicious stock transactions by company executives...READ MORE
- Gilead’s COVID med remdesivir is scarce and costly, AGs say, urging feds to sidestep its patents (fiercepharma.com)
Unhappy with the price and availability of Gilead’s remdesivir—the only drug with FDA clearance to treat COVID-19—dozens of state attorney generals have called for the federal government to exercise march-in rights to allow for broader production of the medicine...In a letter to the heads of the FDA, HHS and NIH, 34 attorneys general wrote that Gilead has been unable to ensure “sufficient” supply and has priced the medicine out of reach for many patients who need it. Gilead is charging $3,120 per treatment course for patients with commercial insurance, Medicare or Medicaid, and $2,340 for patients on certain smaller federal programs...READ MORE
- AllianceRx Walgreens Prime unveils new patented process for delivering specialty medicine (chaindrugreview.com)
A new patented process for delivering specialty medicine will assure AllianceRx Walgreens Prime patients receive their medicine delivered at the correct temperature...It is the only specialty pharmacy to offer a patented cold-chain shipment packaging process...Maintaining the right temperatures is critical to ensuring the efficacy of specialty medications, including costly biologics and injectables, which have special storage or temperature requirements...The new patented process also reduces waste and eliminates reship needs...AllianceRx Walgreens Prime anticipates a significant annual savings in reship costs...READ MORE
- Trump signs executive order to boost U.S. drug manufacturing (reuters.com)
President Donald Trump...signed an executive order aimed at boosting U.S. production of medicines and medical equipment, lowering drug prices and protecting the United States against shortfalls in a future pandemic...Trump said the order would also support advanced manufacturing processes that would benefit U.S. pharmaceutical companies...The long-awaited measure includes a “Buy America” provision mandating federal purchases of certain medical supplies and equipment deemed essential and moves to remove regulatory hurdles to approval of new U.S. drugs...READ MORE
- Study Shows Significant Decline in Cancer Screenings Amid Pandemic (drugtopics.com)
A recent study suggests that fewer patients sought camcer(sic)-related care as a result of the coronavirus disease 2019 pandemic...Published in JCO Clinical Care Informatics...the study...measure(d) the effects of the pandemic on normal cancer care activities, including cancer screening efforts...the most significant findings...relate to cancer screening...declines in mammograms and colorectal cancer screenings...According to the investigators, the data indicate underlying trends observed using diagnosis data and suggest a potential increase in the presentation of later-stage disease for newly diagnosed patients in future months...READ MORE