- Emergent makes history with first FDA nod for over-the-counter naloxone (fiercepharma.com)
...the FDA approved the first over-the-counter naloxone product...the U.S.’ drug regulator blessed Emergent’s 4mg Narcan nasal spray with a nonprescription nod, teeing up direct-to-consumer sales at places like drug stores, convenience stores, supermarkets and gas stations...the timeline on which the product will be made available at stores—as well as its price—is up to Emergent...Other formulations and dosages of naloxone will remain prescription only, though there are access laws that technically permit pharmacies across all 50 states to dispense the emergency drug without a doctor’s note...READ MORE
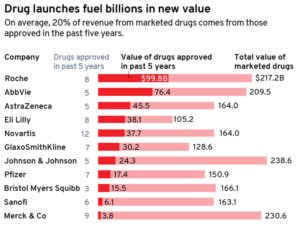
- Who’s getting the most out of their R&D engine? Pharma’s top 11, ranked (fiercepharma.com)
Drugmakers have myriad tools in their arsenal when looking to grow sales. They can acquire marketed drugs, raise prices or focus on growing the reach of their existing medicines. But it's often new drug approvals that reign supreme and ultimately prove the worth of a company's development engine...covered in a recent Evaluate Vantage report, the team at Fierce Pharma took a close look at the recent approvals for 11 of the world's biggest drugmakers by revenue. Specifically, we're highlighting the dollar value of the industry's launches from the last five years and analyzing how the new meds fit into each company's overall portfolio...READ MORE
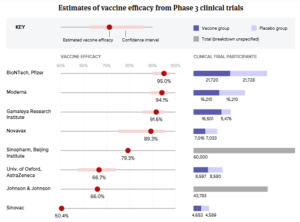
- The first coronavirus vaccines have arrived. Here’s where the rest stand. (biopharmadive.com)
Study results showed vaccines from J&J; and Novavax to be effective against COVID-19. But seemingly weaker protection versus new virus variants have raised concerns...Scientists, drugmakers and governments have moved with unprecedented haste to develop a vaccine against the new coronavirus...The fastest of them have completed studies proving their vaccines can protect against COVID-19. A half dozen shots from developers in the U.S., U.K., Germany, China and Russia have now been cleared by regulators for emergency use...READ MORE
- EMA authorizes Pfizer/Biontech vaccine as new SARS-CoV-2 variant emerges (bioworld.com)Comirnaty (BNT162b2) Vaccine (precisionvaccinations.com)
The EMA has issued a positive opinion on Pfizer Inc./Biontech SE’s COVID-19 vaccine, BNT-162b2, becoming the first regulator to recommend a full marketing authorization, rather than approval for emergency use...The vaccine, now brand named Comirnaty, still has to go through the formality of being approved by EU member state governments, but the EU health commissioner, Stella Kyriakides, has said she expects roll out to start on Dec. 27...“This is the first marketing authorization of a COVID-19 vaccine in the EU. It is valid in all 27 member states at the same time,” said EMA Executive Director Emer Cooke. “There is a firm scientific foundation for roll out,” she said...READ MORE
- COVID-19 pills from Pfizer, Merck authorized by FDA in major pandemic milestone (biopharmadive.com)
Paxlovid and molnupiravir are the first oral treatments for COVID-19, potentially valuable new tools as the fast-spreading omicron variant fuels a sharp surge in cases across the U.S...The Food and Drug Administration...authorized the first pill for COVID-19, clearing for emergency use an antiviral treatment from Pfizer at a precarious moment in the two-year-old pandemic. One day later...the agency cleared a second pill developed by Merck & Co...READ MORE
- J&J Covid-19 Vaccine Authorized for Use in U.S. (msn.com)Johnson & Johnson's COVID-19 vaccine scores FDA authorization, adding key third shot to U.S. supply (fiercepharma.com)
The first single-dose Covid-19 vaccine, a shot from Johnson & Johnson, was authorized for use...the third issued by the U.S. Food and Drug Administration, will give health authorities a desperately needed new source of doses as they scramble to ramp up inoculations ahead of elusive emerging strains...J&J said it has begun shipping the vaccine to the U.S. government, which is managing allocation and distribution. That indicates first doses could be administered during the coming week...READ MORE
- FDA clears Lilly’s COVID-19 antibody cocktail for emergency use (biopharmadive.com)
The Food and Drug Administration...cleared an antibody drug cocktail from Eli Lilly for emergency use for treating people recently diagnosed with COVID-19...The cocktail, a combination of two coronavirus-targeting antibodies, is authorized only for people with mild or moderate symptoms of COVID-19, but who are at high risk of the disease's worst effects due to age, underlying medical conditions or other preexisting conditions...The combination pairs Lilly's bamlanivimab...with another antibody called etesevimab that the drugmaker developed in partnership with China's Junshi Biosciences. Each antibody targets a separate section of the "spike" protein used by the SARS-CoV-2 virus to breach the body's cells — a feature designed to preserve the drug's effectiveness even as the virus mutates...READ MORE
- FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Children Ages 5 to 11 (pharmacytimes.com)
Officials with the FDA have authorized the COVID-19 vaccine from Pfizer and BioNTech for use in children 5 through 11 years of age, based on submitted data and an advisory committee recommendation...the vaccine’s safety was evaluated in approximately 3100 children who received the vaccine. No serious adverse effects were detected in the ongoing study, according to the press release...approximately 8300 cases in children 5 through 11 years of age resulted in hospitalization, and as of October 17, 2021, 146 deaths from COVID-19 have been reported in the United States in this age group...READ MORE
- WHO authorizes AstraZeneca’s COVID vaccine for emergency use (kold.com)
The World Health Organization has granted an emergency authorization to AstraZeneca’s coronavirus vaccine, a move that should allow the U.N. agency’s partners to ship millions of doses to countries worldwide as part of a U.N.-backed program to tame the pandemic...the WHO said it was clearing the AstraZeneca vaccines made by the Serum Institute of India and South Korea’s AstraZeneca-SKBio...“Countries with no access to vaccines to date will finally be able to start vaccinating their health workers and populations at risk,” said Dr Mariângela Simão, the WHO’s Assistant-Director General for Access to Medicines and Health Products....READ MORE
- UK authorizes AstraZeneca, Oxford coronavirus vaccine, but questions linger (biopharmadive.com)
The U.K.'s drug regulator has authorized a coronavirus vaccine from AstraZeneca and the University of Oxford, the first clearance for a shot viewed as critical to global immunization efforts but whose exact effectiveness is uncertain...The Medicines and Healthcare Products Regulatory Agency based its decision on positive data from studies in the U.K., Brazil and South Africa, which showed the shot was able to prevent symptomatic COVID-19 in a majority of vaccinated participants and protect against severe disease. The authorization comes less than a year after a team of Oxford researchers adapted pre-existing work on different coronaviruses to develop a shot for SARS-CoV-2...READ MORE