- Hope for Sarepta drug approval? FDA requests more data (statnews.com)
In the latest twist surrounding Sarepta Therapeutics and its rare disease drug (eteplirsen), the Food and Drug Administration has asked the company to provide additional data to review, a move some see as a sign that the agency is looking for ways to approve the treatment. Shares in Sarepta...jumped more than 25 percent...The request comes amid ongoing uncertainty over the fate of a medicine being developed to combat Duchenne muscular dystrophy, which destroys muscle fibers and eventually confines boys to wheelchairs before sending them to an early death...The controversy increasingly resembles the fracas over HIV drugs three decades ago...the FDA appears caught between its mandate to adhere to scientific standards for approving medicines and finding ways to appease the public and authorize treatments for unmet medical needs.
- Can a pricey implant to treat opioid addiction save lives — and money? (statnews.com)
The implant promises to treat opioid addiction without the hassle of a daily pill. And the company marketing the drug is so confident it’ll work, it’s planning to offer insurers a twist on a money-back guarantee: If the new device doesn’t save them money, they’ll get a refund...The implant, branded as Probuphine, relies on four tiny rods implanted under the skin to dispense the drug buprenorphine for six months at a time. The Food and Drug Administration is expected to decide on Friday whether to approve it...Braeburn Pharmaceuticals...has commercial rights to the implant...plans to price the implant "competitively" with other injectable drugs for neuropsychological conditions…"We are going to put our money where our mouth is," said Braeburn CEO Behshad Sheldon. "We believe that when you guarantee compliance with a medicine, it is going to save money in the long run."...The FDA rejected the implant the first time it came before the agency in 2013, requesting more data demonstrating its efficacy.
- FDA wants drug makers to pay fees to review OTC medicines (statnews.com)
The US Food and Drug Administration is paid fees by the pharmaceutical industry to review brand-name and generic drugs. Now, the agency wants to collect money to review over-the-counter medicines...Specifically, the agency wants companies to pay for reviewing OTC monographs. Unlike brand-name and generic drugs, which involve individual companies seeking approval to market individual drugs, the OTC monographs refer to multiple medicines that share the same ingredient for the same use. Moreover, numerous companies can make these drugs, but do not require FDA approval prior to marketing...the pharmaceutical industry has largely supported so-called user fees...the fees helped transform the relationship between companies and regulators, who are under increasing pressure to meet benchmarks for speeding applications through the review process...Given the workload, the FDA argued that user fees will only bolster its ability to review OTC medicines…the large OTC companies interested in a modernized system who will support it in exchange for some concessions from FDA...The smaller, more fragmented (companies) will probably oppose it as (fees represent) more regulatory burden on them in an area that has worked for decades without fees
- FDA generics backlog improves, although criticism continues (drugtopics.modernmedicine.com)
While many groups are criticizing the FDA's backlog of generic drug approvals, the agency said the situation has improved...In December 2015 alone, OGD (Office of Generic Drugs) issued 99 approvals and tentative approvals...the most approvals and tentative approvals granted in a single month since the start of the generic drug program...FDA’s Office of Generic Drugs awarded 580 generic drug approvals and 146 tentative approvals in 2015...the Campaign for Sustainable Rx Pricing...is pressing legislators to grant FDA more resources, to allow quicker processing of generic drug applications. The group's members include AARP, ASHP, numerous health plans, providers, and Walmart...The FDA faces a backlog of nearly 4,000 generic drug applications, yet approval times can be three or more years...The FDA should be provided necessary resources to clear this backlog and prioritize generic drug approval applications...
- Heavyweight champion of the world (pharmatimes.com)
For more than 15 years NICE has punched above its weight internationally but as it comes under attack for its methodology, will its international clout suffer?...The bad headlines are back; on 20 May, the National Institute for Health and Care Excellence...came under fire from the media for its decision to bar Roche's Perjeta (pertuzumab) for breast cancer from the NHS...While patient groups and charities are worried about the effect on cancer patients in England, who may be denied treatment, there may also be a knock-on effect in other countries, which still look to NICE for guidance over their own reimbursement decisions...NICE International...advises countries from Ghana to Kazakhstan on HTA (health technology assessment) methodology and implementation...Yet, its international spread has come hand-in-hand with the growing disillusionment about NICE's decisions...An IMS Institute study in 2013 compared the reimbursement of cancer drugs in five countries that used cost per QALY (NICE's quality-adjusted life years) methodologies, including the UK, with five using broader methodologies, including Germany and US. It found that the cost per QALY countries reimbursed fewer cancer drugs, had slower access to those they did adopt, and generally performed poorer in terms of cancer outcomes. Moreover, it was not clear that they saved much money.
- Senators urge FDA to approve Sarepta drug for Duchenne (statnews.com)FDA delays decision on whether to approve Sarepta drug for Duchenne (statnews.com)
As a crucial deadline nears for a closely watched regulatory decision, two Republican senators are urging the Food and Drug Administration to approve a controversial drug to treat Duchenne muscular dystrophy...In a letter sent last Friday, the senators expressed "disappointment" that an FDA advisory panel last month voted not to recommend eteplirsen to combat the disease, which is a rare and fatal genetic disorder that causes muscles to waste away...The panel determined the drug was not effective...The FDA is scheduled to decide whether to approve the drug...by this coming Thursday...its decision is being closely watched as a litmus test for the agency, which is grappling with increasingly assertive patient groups that want the agency to take a more expansive view toward approving medicines for unmet medical needs...The advisory panel, which consisted of scientists and doctors who are not FDA employees, voted seven to three, with three abstentions, against recommending approval. But asked whether accelerated approval should be granted, the vote was much closer vote: seven said no and six said yes. The agency is not obligated to accept panel recommendations, but usually does so.
- Ex-FDA head and Sanofi call for harmonized drug regulation (reuters.com)The need for global regulatory harmonization: A public health imperative (stm.sciencemag.org)
Drug regulation has failed to keep up with a globalized world and governments should harmonize oversight to improve patients' access to new and innovative medicines...That is the view of the former leader of the U.S. Food and Drug Administration, the world's top drug regulator, and the research head of French drugmaker Sanofi, who made a joint plea to governments for action...Margaret Hamburg, who led the FDA until 2015, and Elias Zerhouni said there was an urgent need to harmonize a "mosaic of regulations" in different countries, and they called for the issue to be taken up at the G8 or G20 groups of nations..."Essentially, it is a hidden bureaucratic inefficiency tax on the whole effort of finding new and valuable therapies," he said in a telephone interview...Drug development is global and we need to have safety and efficacy data globally, so we should have a global system, just like with airplanes...Sanofi was spending around 20 percent of its research and development budget on ensuring convergence between different systems, often involving duplication of efforts.
- Debate over Duchenne drug hits fever pitch as D-day approaches (fiercebiotech.com)
The deadline for a final marketing decision at the FDA on Sarepta’s Duchenne muscular dystrophy drug eteplirsen arrives on Thursday. And the lead up to D-day continues to see patient advocates and their supporters passionately squaring off against some prominent experts who believe that an approval based on data regulatory insiders have deemed woefully inadequate would create a dangerous precedent at the FDA...Quite a few analysts give Sarepta only a 15% to 20% chance of success in gaining an accelerated approval this week, and that figure would likely be much lower if not for the powerful lobbying campaign that has enlisted the support of some prominent newspapers and politicians...The FDA's job is to get drugs out on the market that are proven safe and effective...there simply were not enough data in place from the corporate sponsor to make that possible. At the same time, companies shouldn't be deluding patients and families into thinking that they have enough data to go to the FDA. They shouldn't approach the FDA unless they truly have the available data to get approved. Relying on patient testimonials and lobbying is not the path to drug approval...There’s only one thing that’s absolutely certain at this point: Whichever way the FDA turns, there will be a storm of protest.
- UK cancer charities call for NICE reform (pharmatimes.com)
Leading UK charities have written to Prime Minister David Cameron urging a review of the system for commissioning drugs in England and Wales which, they argue, has put thousands of cancer patients at risk of missing out on the most innovative therapies...In an open letter to the Prime Minister...the heads of leading cancer charities warn that plans to leave the appraisal methodology employed by the National Institute for Health and Care Excellence unchanged will soon lead to effective new cancer medicines struggling to gain approval...Under current government proposals the Cancer Drugs Fund’s assessment of medicines will be handed back to NICE..."We must not forget the CDF was established as an emergency measure to bypass the very NICE appraisal process to which it is now returning because it was not working for cancer patients," the charities said, and called for "a sustainable system, flexible enough to ensure that the best cancer drugs can routinely benefit NHS patients"...With an anachronistic NICE system still unable to engage in price negotiation, it’s inevitable that patients will not be able to access the clinically-proven medicines that could mean so much to them...it is "high time" for a review of the system.
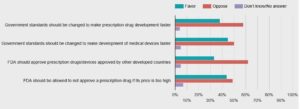
- Public wary of faster approvals of new drugs, STAT-Harvard poll finds (statnews.com)
A majority of Americans opposes federal regulatory changes to speed up the development and approval of new medical treatments, a new STAT-Harvard poll finds — suggesting the public has serious doubts about legislation now moving through Congress...both parties are pushing to change government regulatory standards that they blame for slowing the approval process to get new products to patients...nearly 6 out of 10 Americans said they oppose changing government safety and effectiveness standards to allow for faster approvals of new prescription drugs by the Food and Drug Administration, while 38 percent said they’re in favor of speedier FDA action...The poll sheds new light on how Americans balance their priorities between speed and safety in the approval of new medical treatments.