- Samsung and Biogen win first EU approval for an Enbrel copycat (fiercebiotech.com)Samsung Bioepis Enters the European Biopharmaceutical Market with Benepali®, the First Fusion Protein Biosimilar Approved by the European Commission (finance.yahoo.com)
A joint venture between Biogen and South Korean giant Samsung won Europe's first approval for a lower-cost version of Amgen and Pfizer's blockbuster Enbrel, planning to launch its injection in the coming weeks...The two companies, doing business as Samsung Bioepis, convinced European regulators to clear their Benepali for all of Enbrel's approved indications, including rheumatoid arthritis, psoriatic arthritis, spondyloarthritis and plaque psoriasis…As for Amgen...believes its hold on Enbrel's U.S. rights will keep it safe from a biosimilar challenge in the coming years. In 2011, Amgen secured new patents related to the antibody that the company says will protect the treatment from competition through 2029. Novartis, leading the charge among Enbrel biosimilars developers in the U.S., is hoping to win approval for its version of the treatment this year while mounting a legal challenge on those patents.
- Critics continue pounding 21st Century Cures Act for threatening patient safety (fiercehealthcare.com)The last word: Will 21st Century Cures Act harm patient safety? (medicaleconomics.modernmedicine.com)21st Century Cures: What You Need to Know (energycommerce.house.gov)
Opponents argue that drugs, devices will be less safe if legislation eases FDA approval rules...Opponents of the 21st Century Cures Act, which is intended to accelerate the transfer of scientific advances in genetics into treatment for patients, say the legislation will threaten patient safety by easing FDA rules intended to protect patients from unproven therapies...Critics argue looser FDA rules will result in drug approvals without the level of rigorous testing currently required...new drugs and medical devices will be less safe and effective and cost more, and that the bill sacrifices long-term value to public health.
- It’s official: FDA shoots past 2014’s new drugs record with Roche lung cancer med nod (biopharmadive.com)
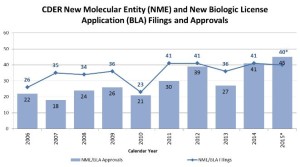
FDA last Friday approved Alecensa (alectinib) for the treatment of ALK-positive non-small cell lung cancer for patients with the disease refractory to therapy with Xalkori (crizotinib). The approval comes well before the drug's March 2016 PDUFA date...This marks the agency's 42nd newly approved medication this year, topping a banner 2014 that saw 41 new drug approvals...there will be a continuing flurry of drug approvals over the next four years (225, to be exact, and most of them in cancer-related therapeutic spaces).
- China toughens drug quality standards, rejects 13 applications (reuters.com)
China's food and drug regulator said late on Monday it had rejected applications for 13 new drugs, citing false or incomplete trial data, as the government toughens enforcement of quality standards…China Food and Drug Administration last month also rejected applications by eight Chinese companies for inadequate trial data related to generic drugs for heart problems, schizophrenia, pain, infections and other diseases…quality of locally made drugs is a priority for the government, which is pushing an ambitious program of healthcare reforms to reduce reliance on both generic and more innovative imported drugs.
- 2015: Another Strong Year for Patients in Need of New Drug Therapies (blogs.fda.gov)Novel New Drugs Summary 2015 (fda.gov)I’m (John Jenkins,Director of the Office of New Drugs) pleased to report another strong year for FDA approvals of novel new drugs, which offer many patients new treatment options for serious and life-threatening conditions. In 2015, FDA’s Center for Drug Evaluation and Research approved 45 novel new therapies – significantly more than the average of 28 we have approved during the previous nine years of this decade...During this past year, we approved many new drugs to treat various forms of cancer, including four to treat multiple myeloma, and others to treat lung, skin, breast, brain, colorectal, and other cancers. We also approved new drugs to treat heart failure, high cholesterol, cystic fibrosis, and irritable bowel syndrome, as well as the first approved reversal agent for a commonly-used blood thinner...Here are a few highlights of these approvals:
- More than one-third of the novel new drugs CDER approved in 2015 were identified by FDA as “first-in-class,” for example, drugs that use a new and unique mechanism of action for treating a medical condition;
- More than 40% of these new therapies were approved to treat rare or “orphan” diseases that affect 200,000 or fewer Americans–Americans who often have few or no drug treatment options;
- 60% of CDER’s novel new approvals for 2015 were designated in one or more categories of Fast Track, Breakthrough, Priority Review, or Accelerated Approval. Each of these designations helps speed the development and/or approval process and is designed to help bring important medications to the market as quickly as possible; and
- 64% of CDER’s novel new approvals were approved first in the United States before any other country.
- DEA eases requirements for natural cannabis-derived drug research (reuters.com)
Drug Enforcement Administration...relaxed some restrictions on research evaluating cannabidiol, an extract of the marijuana plant, for medicinal use...The modifications will ease some requirements imposed by the Controlled Substances Act on possession of cannabidiol (CBD) for a specific Food and Drug Administration approved research protocol...researchers who expanded the scope of their studies and required more CBD than initially approved had to request, in writing... the changes...a previously registered CBD clinical researcher who is granted a waiver can readily modify the protocol and continue research seamlessly. (waiver effectively removes a step from the approval process)...A handful of companies are developing cannabis-derived drugs. Pioneering the effort is Britain's GW Pharmaceuticals, which is slated next year to deliver the results of four late-stage U.S. studies of its botanical pot-based epilepsy treatment...INSYS Therapeutics Inc and Zynerba Pharmaceuticals Inc are working on much earlier stages of development with synthetic cannabis for a number of disorders.
- FDA approves first chemo antidote (pharmatimes.com)FDA Approval of VISTOGARD®, the First Antidote to Treat Overdoses and Early-Onset Severe Toxicities Due to 5-Fluorouracil and Capecitabine Chemotherapies (wellstattherapeutics.com)
US regulators have issued a speedy green light for the first and only drug able to treat chemotherapy overdose or severe allergic reactions...The decision allows doctors to use Wellstat/BTG’s Vistogard (uridine triacetate) to treat patients following an overdose of 5-fluorouracil or capecitabine, or in patients exhibiting early-onset, severe or life-threatening toxicity affecting the cardiac or central nervous system...In combination with other drugs or radiation, 5-FU is a mainstay of chemotherapy across various solid tumours, and capecitabine is a prodrug of the chemotherapy that is enzymatically activated within the body and transformed into 5-FU...The approval of Vistogard is important because it represents the first treatment with a demonstrated track record of efficacy and, just as important, it allows some patients to resume chemotherapy sooner following the resolution of the toxicity...
- Drug approvals at 19-year high belie industry challenges (reuters.com)
2015 was a good year for innovation in medicine with the Food and Drug Administration approving 45 novel drugs, four more than in 2014 and the most since the all-time record of 53 set in 1996...the European Medicines Agency recommended 93 new products, including generics, up from 82 in 2014...the prospect for further progress in 2016, the pharmaceuticals industry faces challenges, with increased political focus on drug pricing having punctured both biotech and specialty pharma valuations in recent months...The rapid pace of new approvals reflects accelerated review times by regulators, who want to get life-saving treatments to patients, especially in cancer, as well as an improved scientific understanding of diseases...Full drug pipelines at many companies suggest the strong rate of new drug launches is likely to continue for a while yet, with IMS Health forecasting a total of 225 new drug approvals between 2016 and 2020.
- 2015: The Year in Specialty Drugs (specialtypharmacytimes.com)
SPECIALTY PHARMACEUTICALS featured prominently in the FDA’s new drug approval and expanded indications list once again in 2015. Below is the first of a 2-part summary of specialty pharmacy–related FDA approvals and expanded indications that took place this year...Part 2, which will be featured in the next issue of Specialty Pharmacy Times, will include oncology drugs and late-breaking FDA actions...
Bleeding Disorders
- Ixinity (coagulation factor IX (recombinant), Emergent BioSolutions Inc)
- Nuwiq (coagulation factor VIII (recombinant), Octapharma)
- Coagadex (coagulation factor X (human), Bio Products Laboratory Ltd)
- Adynovate (antihemophilic factor [recombinant] pegylated, Baxalta Inc)
Inflammatory Conditions
- Cosentyx (secukinumab, Novartis Pharmaceutical Corp.)
- Humira (adalimumab, AbbVie)
Cystic Fibrosis
- Kalydeco (ivacaftor, Vertex Pharmaceuticals)
- Orkambi (lumacaftor/ivacaftor, Vertex Pharmaceuticals )
HIV
- Evotaz (atazanvir/cobicistat, Bristol-Myers Squibb Co)
- Prezcobix (darunavir/cobicistat, Janssen Therapeutics)
- Genvoya (cobicistat/elvitegravir/emtricitabine/tenofovir alafenamide, Gilead Sciences, Inc)
Hepatitis C
- Technivie (ombitasvir/paritaprevir/ritonavir, AbbVie Inc)
- Daklinza (daclatasvir, Bristol-Myers Squibb)
- Harvoni (ledipasvir/sofosbuvir, Gilead Sciences, Inc.)
Multiple Sclerosis- Glatopa (glatiramer acetate injection, Sandoz, Inc.)
- Betaconnect (Bayer HealthCare’s electronic auto injector - Betaseron (interferon beta-1b)
Specialty Ophthalmics
- Lucentis (ranibizumab, Genentech)
- Eylea (aflibercept, Regeneron Pharm)
Hypercholesterolemia
- Praluent (alirocumab, Sanofi-Aventis)
- Repatha (evolocumab, Amgen Inc)
Miscellaneous Specialty Approvals
- Natpara (parathyroid hormone, NPS Pharmaceuticals, Inc)
- Cresemba (isavuconazonium sulfate, Astellas Pharma US, Inc)
- Cholbam (cholic acid, Asklepion Pharmaceuticals LLC)
- Jadenu (deferasirox, Novartis Pharmaceuticals)
- Anthrasil (anthrax immune globulin intravenous [Human], Cangene Corp)
- Rapamune (sirolimus, Wyeth Pharmaceuticals)
- Promacta (eltrombopag, GlaxoSmithKline and Novartis Pharmaceuticals)
- Envarsus XR (tacrolimus extended-release tablets, Veloxis Pharmaceuticals A/S)
- Dysport (abobotulinumtoxinA, Medicis and Ipsen)
- Keveyis (dichlorphenamide, Taro Pharmaceutical Industries Ltd)
- Procysbi (cysteamine bitartrate, Raptor Pharmaceutical)
- Xuriden (uridine triacetate, Wellstat Therapeutics Corporation)
- Reefer Gladness? DEA OKs Catalent to supply marijuana from Missouri plant (in-pharmatechnologist.com)
Catalent has registered a facility in Missouri with the DEA to import cannabis extracts in dosage form for clinical trial studies…The contract development and manufacturing organisation applied for its Kansas City...site to be registered as an importer of controlled substances in August, and last week the Drug Enforcement Administration approved the request…“[Catalent Pharma Solutions] is granted registration as an importer of marihuana, a basic class of controlled substance listed in schedule I,”…“The company plans to import finished pharmaceutical products containing cannabis extracts in dosage form for clinical trial studies.”..One of Catalent’s customers is GW Pharmaceuticals which has a marijuana-based compound, Epidiolex (cannabidiol), in Phase III trials for the treatment of Lennox-Gastaut syndrome…The company ships finished product to a storage facility run by Catalant in the US and investigators draw material from that facility,”