- Nine Explanations For Why The FDA Is Approving Almost Every New Drug Application (forbes.com)The drug development and approval process is about much more than the final “okay” (catalyst.phrma.org)
..Food and Drug Administration, which once approved as few as 40% of new drugs submitted by industry, has been on a green-light-almost-everything jag, approving 89% of drug applications. What’s more, a closer look showed an even higher approval rate. This year so far, 96% of new molecular entities.. – that have been submitted to the FDA have reached the market. For anyone who was watching the FDA a decade ago, that’s just shocking. Good or bad, it’s a radical change...there are a lot of factors that explain why the FDA approval rate is suddenly so high,..
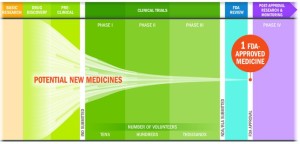
- The approval rate is much lower, because only 12% of drugs that enter clinical trials reach the market.
- Drug companies are better at research, and they are simply producing better drugs.
- Drug companies are picking areas where the chances of approval are higher.
- The FDA is doing a better job communicating with companies.
- The FDA has more power to restrict the use of an approved drug than it used to.
- The FDA is taking a risk by taking strong stands against drug approvals right now.
- The FDA is without a permanent commissioner.
- It’s just random chance.
- In the current political environment, the agency is approving drugs it shouldn’t.
- FDA approves first treatment for sexual desire disorder (fda.gov)Approved Risk Evaluation and Mitigation Strategies (REMS) (accessdata.fda.gov)
Addyi is being approved with a risk evaluation and mitigation strategy, which includes elements to assure safe use…because of the increased risk of severe hypotension and syncope due to the interaction between Addyi and alcohol…REMS requires that prescribers be certified… Additionally, pharmacies must be certified…Certified pharmacies must only dispense Addyi to patients with a prescription from a certified prescriber...
- Raleigh’s Sprout Pharmaceuticals awaits FDA ruling on female libido drug (newsobserver.com)
Sprout Pharmaceuticals is a small drug company with a potentially very big drug – the world’s first pill to boost women’s sex drive….Food and Drug Administration is expected to announce Tuesday whether it has approved Sprout’s drug, Addyi (flibanserin), ..plans to market as the "little pink pill"… Interest in Sprout’s libido pill is expected to run so high that, to curb potential misuse, Sprout has promised the FDA it won’t advertise the drug for 18 months on TV and radio.
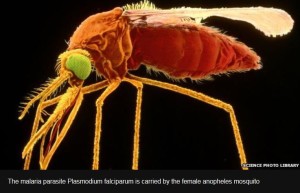
- Malaria vaccine Mosquirix gets European approval (cbc.ca)
first malaria vaccine got a green light... from European drugs regulators who recommended it as safe and effective to use in babies in Africa at risk of the mosquito-borne disease....called RTS,S or Mosquirix, and developed by GlaxoSmithKline in partnership with the PATH Malaria Vaccine Initiative, would be the first licensed human vaccine against a parasitic disease and could help prevent millions of cases of malaria...
- Generic Drug Sponsors Will Get Status Reports– But Only For Old ANDAs (pharmamedtechbi.com)
FDA is ready to restore some informal communications for generic drugs, but only for applications not covered by the newly implemented user fee action dates…applicants with pending ANDAs (abbreviated new drug application) that are not covered by user-fee goals will have the right to a status report under a new policy …governing communications with ANDA applicants in response to widespread criticism from the generic industry about the loss of interaction that followed enactment of the Generic Drug User Fee law..
- F.D.A. Approves Addyi, a Libido Pill for Women (nytimes.com)FDA approves first treatment for sexual desire disorder (fda.gov)
first prescription drug to enhance women’s sexual drive won regulatory approval …victory for a lobbying campaign that had accused the Food and Drug Administration of gender bias for ignoring the sexual needs of women…Addyi (flibanserin) — is actually the first drug approved to treat a flagging or absent libido for either sex. Advocates who pressed for approval…Even the Score, said that a drug to improve women’s sex lives was long overdue… National Consumers League..the campaign behind Addyi … made a mockery of the system that regulates pharmaceuticals..
- FDA Unveils User Fee Rates for FY2016 (raps.org)
1980s, the US lagged behind Europe in drug approvals, and individual drug reviews often took years to complete. The problem, FDA argued, lacked adequate funding to hire the staff it needed to review drugs in a more timely manner….solution FDA proposed was to collect one-time fees from the companies it regulates…. led to…Prescription Drug User Fee Act…
- Drugs just don’t get rejected much anymore, report says (fiercebiotech.com)
Picking apart biopharma’s protracted boom,…the vibe that getting drugs approved is simply much easier than it once was…. FDA has been green-lighting new drugs at an escalating rate for the past few years… some think the agency can go farther… 21st Century Cures Act,..contains a bevy of provisions designed to bring medicines to the market more quickly…The bill has faced staunch criticism from public health officials and media outlets, cautioning that there can be too much of a good thing, and improperly evaluated drugs can be just as dangerous to patients as no treatments at all.
- FDA approves use of opioid painkiller in 11-16 year olds (reuters.com)CDER Conversation: Pediatric pain management options (fda.gov)
Food and Drug Administration has approved the use of opioid painkiller OxyContin in patients aged 11 to 16 who have not benefited enough from alternatives….warnings and precautions for pediatric patients are the same as those for adults...
- FDA Clears First 3D-Printed Drug (Spritam [Levetiracetam]) (medscape.com)Aprecia Preps to 3D Print Medicine with New Facility (video) (3dprintingindustry.com)
Food and Drug Administration has approved the first three-dimensional printed oral drug product, Spritam (levetiracetam), from Aprecia Pharmaceuticals,…indicated as adjunctive therapy for partial-onset seizures, myoclonic seizures, and primary generalized tonic-clonic seizures in adults and children with epilepsy…was developed with Aprecia's proprietary ZipDose technology, which uses three-dimensional printing to create a porous formulation...that disintegrates rapidly...






![FDA Clears First 3D-Printed Drug (Spritam [Levetiracetam]) (medscape.com)](https://nevadapharmacytoday.com/wp-content/uploads/2015/08/zip-dose3-300x176.jpg)