- Health Canada approves ‘Viagra for women’ pill, loosens restrictions on taking it with alcohol (edmontonjournal.com)
In Canada, the libido pill will come with a warning to ‘limit’ alcohol consumption, in contrast to the U.S., where women must promise they’ll abstain while on the drug...Canada has approved the first “Viagra for women” pill — and, to the alarm of some experts, loosened restrictions around alcohol that have contributed to the drug’s lacklustre sales in the United States...In Canada, the libido pill will come with a warning to women to “limit” their alcohol consumption...The drug had been under review by Health Canada for more than two years. The federal agency issued a “notice of compliance” late last month...The U.S. Food and Drug Administration twice rebuffed Addyi before granting approval in 2015, and only after lobbying from a “grassroots” campaign called Even the Score — funded by Addyi’s makers, Sprout Pharmaceuticals — that accused the regulator of being sexist for approving sex medicines for men, but not for women...
- FDA Delays Implementing Parts of ‘Intended Use’ Rule (raps.org)
Confusion and concerns over portions of a tobacco-related final rule have pushed the US Food and Drug Administration to delay implementation of the sections dealing with the types of evidence FDA may consider to determine how a manufacturer intends for its medical product to be used...This is a determination that can have significant implications for, among other things, how manufacturers communicate about and promote their products. How we determine intended use is an important issue that touches many fundamental aspects of the FDA’s work...FDA is seeking comments on the proposal to delay...effective date of the portion of the rule related to intended use of medical products, though the tobacco-related portions of the final rule will go into effect...At issue is how the initially proposed rule sought to remove a sentence, which FDA Law Blog last February called "the famous 'knowledge' sentence: 'But if a manufacturer knows, or has knowledge of facts that would give him notice, that a [drug or device] introduced into interstate commerce...is to be used for conditions, purposes, or uses other than the ones for which he offers it, he is required to provide adequate labeling for such a drug/device which accords with such other uses to which the article is to be put.'"...But the final rule did not remove the sentence and instead amended it with new language that angered industry groups, which called the move "a new and unsupported legal standard."
- EMA Updates Brexit Guidance (biopharminternational.com)
The agency and the European Commission published updated guidance to answer questions about Brexit...On Dec. 1, 2017, the European Medicines Agency and the European Commission published updated guidance for pharmaceutical companies regarding the United Kingdom’s withdrawal from the European Union. The new guidance answers additional questions about marketing applications and authorizations...New in this update is more information on batch release sites located in the UK, which must be located in the EU; the effect on herbal medicinal products; and applications for orphan drug designation. The guidance also discusses the local representatives in the UK mentioned in product information, global marketing, and the sunset clause.
- FDA Expands Generic Drug Priority Reviews (raps.org)
Talk of bringing down the price of pharmaceuticals often hinges on generic competition, and the US is seeing approvals of new generic drugs faster and more consistently than ever – a trend likely to continue...The progress comes as Food and Drug Administration Commissioner Scott Gottlieb...indicated that the agency will expand which abbreviated new drug applications will see priority reviews..."Earlier this year we made changes to how we prioritize the agency’s generic drug submissions. The goal was to prioritize the review of generic applications until the FDA has approved three generic versions of each particular drug," Gottlieb said in a statement. "Today we’re expanding this competition-focused policy to prioritize any application that can meet the FDA’s approval standards at the point when the 180-day exclusivity period expires on a first generic entrant to a branded medicine."...The shift could accelerate generic competitors to market more quickly and help bring down costs, and comes a day after the Federal Trade Commission held a workshop on drug competition.
- Numbers show drugmakers are keeping RTFs under wraps (biopharmadive.com)
When Celgene Corp. revealed receipt of a Refusal-to-File letter for its blockbuster hopeful multiple sclerosis drug ozanimod, shares in the big biotech dropped as much as 10%...The decline is no surprise — such a major disclosure was bound to hit Celgene's stock price because the Food and Drug Administration's decision not to accept the New Drug Application will significantly impact the time when (and if) ozanimod ever gets to market, potentially costing Celgene hundreds of millions in future sales...But not all pharmas seem to be so upfront with shareholders about the letters from the FDA, according to a BioPharma Dive analysis...Public disclosure therefore falls to the company when it receives one of these rejections. Company executives can disclose as much or as little information about the letters as they deem appropriate...There has been controversy over the issue for years related to CRLs (Complete Response Letters) — and public and investor outcry for the FDA to publish the letters...it comes down to what is "material" to investors, something defined by the Securities and Exchange Commission but which also gives companies some latitude...
- New drug approvals hit 21-year high in 2017 (reuters.com)
U.S. drug approvals hit a 21-year high in 2017, with 46 novel medicines winning a green light -- more than double the previous year -- while the figure also rose...in the European Union...recommended 92 new drugs including generics, up from 81...Yet the world’s biggest drugmakers saw average returns on their research and development spending fall, reflecting more competitive pressures and the growing share of new products now coming from younger biotech companies...projected returns at 12 of the world’s top drugmakers were at an eight-year low of only 3.2 percent...
- OTC Viagra: Pfizer snags nod for nonprescription sales of the little blue pill for men in the U.K. (fiercepharma.com)
Pfizer has won a first approval for OTC Viagra. Viagra Connect, the Pfizer OTC name for its blockbuster erectile dysfunction drug, has been approved for sale in the U.K...The Medicine and Healthcare products Regulatory Agency announced...that it will reclassify the 50 mg dose from prescription only to a pharmacy medicine in the U.K. Viagra Connect is expected to be available for sale in the spring of 2018. Anyone seeking to buy the drug will be required to have a discussion with a pharmacist, who will determine whether the drug is appropriate for their use...It will continue to sell branded versions of other doses of the drug in the U.K...In regard to its U.S. OTC ambitions, Pfizer said, in a statement to FiercePharma, “While we do not have information to share on specific Rx to OTC switch programs in the United States, generally we consider prescription drugs—both within the Pfizer portfolio and outside it—for potential switch to non-prescription status. Our objective is to provide consumers with significantly greater access to medicines with well-established efficacy and safety profiles without a prescription.”
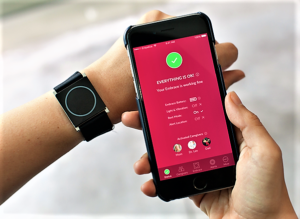
- FDA Clears the First Smart Watch for Use in Neurology (ptcommunity.com)
Wearable device identifies convulsive epileptic seizures and sends alerts to caregivers...The FDA has cleared the Embrace smart watch (Empatica, Inc.) for use by patients with epilepsy. Embrace uses advanced machine learning to monitor for the most dangerous kinds of seizures, known as “grand mal” or “generalized tonic-clonic” seizures, and sends an alert to summon caregivers’ help...The smart watch stands apart from any seizure detection system in that it measures multiple indicators of a seizure. Its unique property is its use of electrodermal activity, a signal used by stress researchers to quantify physiological changes related to sympathetic nervous system activity, also known as the "fight-or-flight" response. Embrace has been approved in Europe as a medical device for seizure monitoring and alert since April 2017.
- 2017 was a big year for FDA digital health regulations (healthcareitnews.com)
With a new administrator at the helm, the U.S. Food and Drug Administration took steps toward regulating decision support, software-as-a-medical-device, mobile tech in clinical trials and more...the FDA had a full plate in 2017 as it sought to revise its regulatory processes for the shifting healthcare landscape...the new guard already had announced and implemented a firm-focused pre-certification program, released new guidances addressing provisions of 2016's 21st Century Cures Act, and outlined a handful of other ongoing initiatives that are sure to impact the digital health industry. Here's a rundown of the agency's actions and announcements during 2017.
- New leadership, new approaches
...a plan that included clear language on which devices the agency would look to regulate, an app regulation strategy involving postmarket data collection, and other idea designed to streamline the approval process...FDA opened up applications for a pilot of a firm-focused digital health pre-certification program...nine companies selected to participate: Apple, Samsung, Verily, Pear Therapeutics, Tidepool, Phosphorus, Fitbit, Roche, and Johnson & Johnson...draft of the long-awaited and somewhat controversial guidance on clinical decision support, which laid out the forms of clinical decision support that would or wouldn't be regulated based on the degree of human involvement (as opposed to risk)...draft guidance describing the FDA's new Breakthrough Devices Program...would supersede the Expedited Access Pathway and aims to push novel technologies presenting a significant improvement over status quo through the clearance process more quickly.
- FDA shifts toward digital, patient feedback
...the Clinical Trials Transformation Initiative – a public-private partnership of pharma companies, academics, and regulators including the FDA – released new endpoint recommendations for the use of mobile technologies in clinical trials... The guidelines, meant to be the first in a series of such documents, included suggestions for study designers when selecting novel endpoints, practical approaches when developing these endpoints...
- New leadership, new approaches
- Europe’s drug industry waits for white smoke in Brussels (reuters.com)
It may be a cross between the Eurovision Song Contest, a papal conclave and a social club raffle but a ballot among EU ministers...could hurt Europe’s pharmaceutical industry and the health of millions...It will fix the new home of the European Medicines Agency, which must leave London by 2019 when Britain leaves the European Union; most of its 900 staff may refuse to move to many of the 19 cities in the running, the EMA warns. Replacing them would delay drug approvals and patient safety checks...Yet the result, diplomats agree, is utterly unpredictable; months of horse-trading on issues unrelated to healthcare will end up in hours of haggling between secret ballots in Brussels...It could even come down to drawing lots...Senior officials liken the process to Europe’s annual TV music schlock-fest, when the winner of the Eurovision Song Contest is often determined by viewers phoning in votes for acts from like-minded neighbouring states and historic allies...British bookmaker Ladbrokes has Milan the 2-1 favorite to secure the EMA...seasoned diplomats hesitate to quote odds: “The most likely result is one that will be perverse,” said one, recalling previous upsets behind closed doors...Another referred to closeted cardinals electing popes at the Vatican: “In the end,” he said, “We will get the white smoke.”