- Is FDA Going Too Far in Enforcing Drug Compounding Law? (c.ymcdn.com)
...the FDA is overstepping its authority and jeopardizing patient access to compounded medications...The pharmacies say the Food and Drug Administration has misinterpreted Congress’s intent when it enacted the Drug Quality and Security Act (DQSA)...by not allowing office-use compounding...The pharmacies also are concerned that the FDA has issued no rules for compounding but only guidance documents, which aren’t legally enforceable...‘Mass confusion has resulted from the usage of guidance documents, and the FDA is definitely circumventing the Administrative Procedure Act (APA) in our opinion,’’ Cynthia Blankenship, executive vice president of the International Academy of Compounding Pharmacists (IACP)...The APA governs how federal agencies propose and establish regulations. IACP wants Congress to pass a bill (H.R. 2871) they say will fix these issues...Since the DQSA was enacted, the FDA has significantly..increased its inspections of drug compounding...facilities...The FDA has conducted more than 350 inspections of compounding pharmacies as of Nov. 27, 2016, and nearly 120 of those inspections were due to reports of serious adverse events or product quality issues...
- Continuous Manufacturing: Pfizer, Vertex, AstraZeneca and Others Weigh FDA Plans (raps.org)
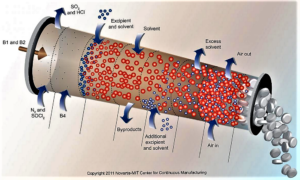
The US Food and Drug Administration has been encouraging the adoption of continuous manufacturing techniques...and several companies recently offered the agency some suggestions to refine its work around the developing technology...continuous manufacturing allows companies to move more seamlessly and efficiently...Last week, FDA finalized guidance on how manufacturers can participate in the agency’s program to advance continuous manufacturing...And in a blog post, Michael Kopcha, director of FDA's Office of Pharmaceutical Quality, pointed to Vertex's cystic fibrosis drug Orkambi (lumacaftor/ivacaftor) and Janssen's HIV treatment Prezista (darunavir) as examples of companies successfully using continuous manufacturing after engaging with FDA's emerging technology team...Vertex Pharmaceuticals...noted several contradictions in how batch size is described in an FDA document and sought further clarity and certainty regarding FDA’s understanding and expectations regarding batch size...AstraZeneca...asked if FDA might consider harmonizing the assessment process of the changes relating to continuous manufacturing, including a mechanism by which there could be some mutual recognition across countries participating in the International Council on Harmonization. AstraZeneca also said it "does not see the need" for a full ICH guideline on continuous manufacturing, which the company says FDA has been supporting. But the European drug industry group known as EFPIA is suggesting a Q&A document based on ICH Q8 and AstraZeneca says it "concurs with that approach."
- ICER Seeks Comments for Proposed Changes for Orphan Drugs Assessments (ajmc.com)
The Institute for Clinical and Economic Review is seeking public comments on its recently released outline of proposed adaptations involving the assessment of certain treatments for ultra-rare conditions, also known as orphan drugs...The proposed changes are intended to provide information for decision makers regarding the practical and ethical challenges involved in potential major advances for ultra-rare conditions. The adaptations are also intended to complement the ICER’s recent update to its value assessment framework...In order to be considered for an adapted approach, a treatment must be expected to be used in a population of less than 10,000 with a minimal chance of future indication, and must demonstrate potential to majorly improve the quality of life or the length of life for the patient...The key statements and proposed changes in the proposal include:
- ICER will continue with the same standard of evidence when rating comparative clinical effectiveness, but will offer context regarding the challenges of producing evidence for the treatments and for identifying data for long-term safety.
- ICER will continue developing cost-effective models for new treatments, but models for ultra-rare conditions will have a broader range of cost-effectiveness results—from $50,000 per quality-adjusted life year (QALY) to $500,000 per QALY—and will calculate a value-based price benchmark from the standard range. However, these reports will indicate that decision-makers often give special weighting to other benefits, causing coverage and funding decisions at higher price—resulting in higher cost-effective ratios for the treatments.
- The report sections "other benefits and disadvantages" and "contextual considerations" will include evidence and perspectives of the potential positive treatment effects on family and the community.
- ICER will conduct a collaborative process to develop a template for information in reports on research, development, and relative costs for ultra-rare condition treatments.
- Endo to pull opioid painkiller off U.S. market after FDA nudge (reuters.com)
Endo International Plc agreed...to remove its long-acting opioid painkiller from the U.S. market to comply with the health regulator's request last month, sending its shares down as much as 3 percent...The U.S. Food and Drug Administration in June had requested the withdrawal of Endo's Opana ER, marking the first time the agency asked for an opioid painkiller to be taken off the market for public health reasons...The regulator's request followed the recommendation of an independent panel of advisers, which concluded that the drug's benefits no longer outweighed its risks...
- U.S. to promote use of opioid alternatives to treat addiction (reuters.com)
The U.S. Food and Drug Administration plans to encourage widespread use among opioid addicts of less harmful opioid drugs such as methadone and buprenorphine, a radical shift in policy that could draw opposition from those in the addiction field who believe abstinence is the only effective treatment...FDA Commissioner Scott Gottlieb outlined a proposal under which every addict who suffers a non-fatal overdose would be treated with an opioid substitute, for long periods if necessary, or even for life...“I know this may make some people uncomfortable,” Gottlieb said...“FDA will join efforts to break the stigma associated with medications used for addiction treatment.”...The FDA, Gottlieb said, will issue guidance for drugmakers to promote the development of new addiction treatments and lay out the agency’s interest in “novel, non-abstinence-based” products...
- Gottlieb vows to shake up the FDA, backing a trend toward faster drug development (endpts.com)
FDA commissioner Scott Gottlieb has sounded a crystal clear warning over the high — and growing — cost of drug development. And in a speech to regulatory execs...Gottlieb committed the FDA to backing up more efficient drug development programs with new measures to clear the regulatory path for developers barreling ahead to relatively swift pivotal data in search of an accelerated OK...Gottlieb started by outlining a bleak picture in drug R&D, noting that the economic model for drug development is broken. It costs too much to develop a drug so it can be approved for marketing. And costs are swelling fast at the discovery end of the business, which will help swamp a system that already doesn’t work particularly well...Gottlieb held up some of the rapid-fire clinical trials we’ve been seeing in the cancer field as a model for what can work, paving the way to the accelerated approval pathway at the FDA. And he believes...that moving drug development into the fast lane can reduce R&D costs and thereby allow biopharma companies to pass on savings to patients through lower costs.
- ‘Unorderly’ Brexit risks shortage of life-saving drugs (pharmaceutical-journal.com)
An ‘unorderly’ Brexit could risk the supply of life-saving medicines to patients because the drugs are held at border checks, piled up in warehouses, or subject to ‘extensive retesting’, the British and European pharmaceutical industries have jointly warned...a disorderly Brexit could result in ‘severe disruption’ of most companies’ supply chains, as the flow of medicines from the UK to the continent and back could stall..."ongoing cooperation" between the UK and EU on medicines as "the best way of ensuring that patients across Europe and the UK are able to continue to access safe and effective medicines and to ensure that there is no adverse impact on public health"...Current European marketing authorisations that apply across the EU, and continued cooperation between national competent authorities that are facilitated by the European Medicines Agency and the European Commission will be important in maintaining effective ties...any changes to the UK–EU trading relationship should not affect the research, development, manufacture and supply of medicines across Europe, including for clinical trials.
- The Other Side of Opioid Limits (drugtopics.modernmedicine.com)
Proponents argue that limits reduce the risk of addiction, but are they keeping pharmacists from caring for their patients?... As the opioid crisis worsens, pharmacies, pharmaceutical manufacturers, and legislators are scrambling to help solve the problem. Recently, those efforts have focused on limiting opioid supplies. But in the effort to prevent unnecessary medications, are pain patients getting left behind?...Express Scripts and CVS Caremark recently announced a seven-day supply limit, and PhRMA...supported a seven-day limit...one-size fits all approach and will supplant providers’ clinical decision-making and the needs of patients who have legitimate need for these medications...payer limits restrict patients with legitimate pain management needs from accessing opioids. Those limitations...will force patients not at risk of abuse or misuse to work with their prescriber and pharmacist—which will cost the health-care system and “significantly” impact patients with limited resources, physical restrictions, or transportation issues...
- F.D.A. Accuses EpiPen Maker of Failing to Investigate Malfunctions (nytimes.com)
The Food and Drug Administration...accused the drugmaker Pfizer of failing to properly investigate reports of malfunctioning EpiPens, including incidents when patients died or became severely ill after the device failed to work. Pfizer manufactures the EpiPen, which treats allergic reactions, for the drugmaker Mylan...In a warning letter...the agency said Meridian Medical Technologies, which is a unit of Pfizer, did not adequately look into problems with a crucial component of the EpiPen — the mechanism on the device that insures that it fires and delivers the proper dose of epinephrine, which stops an allergic reaction... the company failed to conduct a proper investigation even though it received numerous complaints about problems with activating the device. "Our own data show that you received hundreds of complaints that your EpiPen products failed to operate during life-threatening emergencies, including some situations in which patients subsequently died...
- Trump’s FDA Commissioner on Drug Prices, Regulations, Science (bloomberg.com)
Trump vs. Big Pharma: Can He Bring Drug Prices Down?...U.S. Food and Drug Administration Commissioner Scott Gottlieb spoke with Bloomberg News about drug pricing, new medicine and regulations. This transcript of the interview has been edited for clarity and length.
- What’s the FDA’s role to play in drug pricing and what can the agency do, given that it hasn’t traditionally had a mandate to address the issue?
- Is it just small, opportunistic drug companies that are "gaming" the system, or is this something bigger companies do as well?
- What about the rest of the administration? Trump talked a lot about drug pricing on the campaign trail and after, yet we haven’t seen much action other than yours.
- What about an executive order on drug pricing -- there was talk that Trump was going to come out with something. Is that still being worked on?
- What about drugs like EpiPen, would you come out with new rules there to create more competition? [EpiPen, made by Mylan NV, is what’s known as a drug-device combination, where both the medicine and the device that administers it can have patent protections.
- But is it reasonable to assume you’re looking at doing something like this, on these types of devices?
- One of the things we’ve seen from the administration is, get rid of regulation, get rid of regulation, get rid of regulation. How does your philosophy as a regulator –- one of the biggest regulators in the U.S. government –- how does that line up with what the Trump administration has called for?
- Some of the things you’re doing to create more competition among drugs, people could interpret as a loosening of standards. I wonder if you think that’s the case?
- Through the years we’ve seen the agency go through cycles, of pushing drugs out into the market faster, versus being much more conservative about safety. Is the balance at the FDA changing?
- How broadly can you apply new standards or guidelines for drug approval? There’s been talk about it in cancer, but what about other diseases?