- AmerisourceBergen to pay $625 million in U.S. civil fraud settlement (reuters.com)
AmerisourceBergen Corp...will pay $625 million to resolve civil fraud charges over the sale of syringes containing drugs for cancer patients, double billing, and providing kickbacks to doctors...The settlement...boosts AmerisourceBergen’s total payout to $885 million over its repackaging and distribution of pre-filled syringes that were not approved by the Food and Drug Administration...AmerisourceBergen admitted that from January 2001 to January 2014, its Medical Initiatives Inc pharmacy unit in Alabama shipped millions of syringes for patients undergoing chemotherapy that contained drugs prepared in an unsterile environment...Authorities said AmerisourceBergen would harvest “overfill” from the original vials of such drugs as Aloxi, Anzemet, Kytril and generic Kytril, Neupogen and Procrit...That enabled the company to create more doses than it bought, and generate at least $99.6 million of extra profit...
- Nevada prisons drug buyer knew firms opposed execution use (kolotv.com)
Nevada's prisons pharmacy chief says she ordered and obtained lethal injection drugs this year despite knowing drug manufacturers didn't want their products used for executions...Linda Fox's drug purchases allowed Nevada to plan its first execution since 2006 using a never-before-tried three-drug combination...She testified...that she didn't specify the end use when she obtained medications from a third-party supplier, not the drug makers...Fox was pressed by lawyers representing drug companies a day after the state's prisons chief provided sworn testimony about having trouble obtaining drugs for executions...
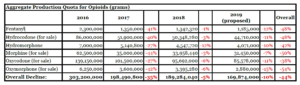
- Trump administration proposes production quota cuts for six opioids (reuters.com)Proposed Aggregate Production Quotas for Schedule I and II Controlled Substances and Assessment of Annual Needs for the List I Chemicals Ephedrine, Pseudoephedrine, and Phenylpropanolamine for 2019 (justice.gov)
The Trump administration on Thursday proposed that U.S. drugmakers cut production quotas of the six most abused opioids by 10 percent next year to fight a nationwide addiction crisis....the U.S. Justice Department and Drug Enforcement Administration said the proposed cut would be in keeping with President Donald Trump’s effort to cut opioid prescription fills by one-third within three years...
- China says vaccine maker Changsheng broke manufacturing rules, faked records: Xinhua (reuters.com)Arrests on the way at Changsheng Bio-tech, the company at the centre of Chinese vaccine scare (scmp.com)China widens vaccine scandal probe, vows tough penalties (reuters.com)
China’s cabinet investigation group has found that vaccine maker Changsheng Bio-technology broke the law in manufacturing rabies vaccines...The investigation group said the company had systematically falsified production and testing records to avoid regulatory scrutiny...“The company used expired materials to produce some rabies vaccine and falsified the production date,”...“To cover up violations, the company systematically fabricated production and testing records.”...China has launched sweeping spot checks on vaccine makers around the country after Changsheng was found to have falsified data and sold ineffective vaccines for children...President Xi Jinping has ordered all relevant departments to investigate the scandal, which has triggered public outrage in what is the latest case of tainted medical products...
- AbbVie’s not the only one facing kickbacks scrutiny. Add Biogen, Sanofi, Gilead and more (fiercepharma.com)
At first glance, it seems like a logical idea: pharma companies teaching doctors' staff how to handle patients using their drugs and helping staffers with reimbursement questions. But a slew of companies are discovering that it's not so simple...In fact, it might just be against the law...Biogen, Sanofi and Gilead Sciences are under investigation by federal and state authorities for offering reimbursement services and clinical education programs, the companies have disclosed in securities filings. Additionally, Bayer, Amgen and Eli Lilly face whistleblower lawsuits over the issue...In Sanofi’s case, the U.S. Attorney’s Office...sent the company a civil investigative deman...“requesting documents and information relating to Sanofi US’s certified diabetes educator program during the period from 2007 to the present.”...Gilead....received a voluntary request from the U.S. Attorney’s Office...seeking information “related to our reimbursement support offerings, clinical education programs and interactions with specialty pharmacies for Sovaldi and Harvoni...
- FDA finds another carcinogen in valsartan products (biopharmadive.com)Updated: FDA, Health Canada and EMA Spot Second Impurity in Valsartan (raps.org)
The FDA's latest round of testing has revealed an additional impurity, N-nitrosodiethylamine (NDEA) in three lots of Torrent Pharmaceuticals' recalled valsartan products. NDEA is a known animal carcinogen and a suspected human carcinogen...The source of the impurity was the valsartan active pharmaceutical ingredient from Zhejiang Huahai Pharmaceuticals. Not all products made from Zhejiang Huahai's valsartan contain the impurity, however...More than half of valsartan products are now under recall and FDA said last month more are likely...The story has now escalated. Once the FDA and EMA learned that Zhejiang Huahai discovered an additional impurity, NDEA, the authorities retested all valsartan API and products. Like NMDA, NDEA appears to be formed as a result of a specific sequence of steps in manufacturing.
- Pfizer joins DOJ probe into claims pharma bribes funded Iraqi terrorists (fiercepharma.com)Veterans' lawsuit claims Big Pharma bribes in Iraq helped finance terrorism (fiercepharma.com)Roche, Johnson & Johnson pulled into Justice Department probe of alleged terrorist bribes (fiercepharma.com)
Pfizer has joined three of its Big Pharma peers in a Department of Justice probe examining allegations that the companies paid bribes to a terrorist-run health ministry in Iraq...The Justice Department's inquiries stem from a lawsuit, filed last fall, in which veterans and their families accused Pfizer, AstraZeneca, Roche and Johnson & Johnson of paying bribes to win business from the Iraqi ministry of health at a time when the ministry was controlled by terrorists...The suit alleges the companies paid bribes to terrorists that "openly controlled the Iraqi ministry in charge of importing medical goods." The plaintiffs contend the drug companies "obtained lucrative contracts from that ministry by making corrupt payments to the terrorists who ran it."
- Health Management Associates to pay $260 million to settle criminal charges for allegedly defrauding Medicare, Medicaid (cnbc.com)Health Management Associates Pays $260 Million To Settle Whistleblower Lawsuits (marketwatch.com)
Health Management Associates agreed to pay more than $260 million and entered a deferred prosecution agreement to settle criminal charges for allegedly paying physicians kickbacks and defrauding Medicare, Medicaid and other federal programs...HMA, which was acquired by the for-profit hospital Community Health Systems in 2014, allegedly paid physicians in exchange for patient referrals and submitted inflated claims for emergency department facility fees to federal health insurance programs...HMA pressured emergency room physicians, including through threats of termination, to increase the number of inpatient admissions from emergency departments — even when those admissions were medically unnecessary...
- Doctor disciplined for looking up Vegas shooter drug records (ktvn.com)Doctor accused of looking up Oct. 1 gunman’s prescriptions keeps license (reviewjournal.com)
A doctor has been disciplined by Nevada state pharmacy regulators after he was accused of improperly looking up prescription records of the dead gunman in last October's mass shooting in Las Vegas...Attorney E. Brent Bryson said Thursday that Dr. Ivan Goldsmith has to pay $26,000 in fines and attorney fees but keeps his license to prescribe medicines if he completes a year of probation...Goldsmith was accused of improperly looking up gunman Stephen Paddock's patient profile and disclosing to the Las Vegas Review-Journal that Paddock had been prescribed diazepam, an anti-anxiety drug better known as Valium.
- Tuesday’s execution in Nebraska the 1st in US to use fentanyl (reviewjournal.com)
Nebraska is preparing to carry out its first execution since 1997 on Tuesday in a bewildering about-face driven largely by the state’s Republican governor...Carey Moore, is scheduled to be executed at the Nebraska State Penitentiary...with a never-before-tried combination of drugs. Moore was condemned to die for the 1979 shooting deaths of...Maynard Helgeland and Reuel Van Ness Jr., and is one of the nation’s longest-serving death row inmates...The combination of drugs for Tuesday’s execution has never been used to put a person to death, according to the Lincoln Journal Star. Three of the drugs — diazepam, fentanyl and cisatracurium — have never been used as part of an execution protocol. The fourth drug, potassium chloride, has been challenged as having the potential to cause serious pain for the inmate.