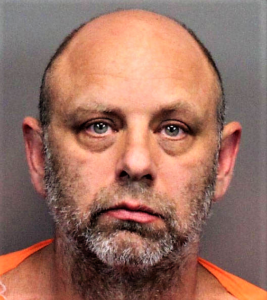
- Reno doctor arrested in April faces new complaint by Nevada medical board (reviewjournal.com)Reno Ford dealer pleads guilty in drug scheme (reviewjournal.com)
A Reno doctor arrested on allegations he participated in an opioid drug ring is the subject of a newly filed complaint by a Nevada medical board alleging 74 violations of the state’s Medical Practice Act...family physician Robert Rand, who was arrested in San Francisco in April, inappropriately treated patients by committing malpractice, violating opioid prescribing standards and engaging in unprofessional conduct, among other accusations...Rand, whose medical license remains active, is currently behind bars, according to the Washoe County Sheriff’s Office...
- Former Lincoln County commissioner sentenced in insurance fraud case (reviewjournal.com)
A former Lincoln County commissioner was sentenced Friday to one-to-four years in prison for defrauding insurance companies...Adam Katschke...previously pleaded guilty to felony insurance and Medicaid fraud in the case...Katschke, the head pharmacist and owner of Meadow Valley Pharmacy in Caliente, defrauded insurance companies...by billing for large amounts of pharmaceutical prescriptions that were rarely provided as billed to the patients or prescribed by a physician...The sentencing...ordered Katschke to pay $1.5 million in restitution...The defendant stole a million and a half dollars from taxpayers through Medicaid, a program designed to provide care for those in need, not line the pockets of fraudsters…
- FDA warning letter tells Clorox to clean up Aplicare plant (fiercepharma.com)
The FDA says the unit of Clorox that manufactures povidone-iodine drug products needs to clean a big mess at a plant in Meriden, Connecticut, which failed to follow steps to insure the sterility of its wound products—products that the FDA also said are “unapproved.”...The agency...posted a warning letter for the Aplicare plant…(it) said...the plant failed to implement adequate microbial controls...some of its products are unapproved because they do not comply with the FDA’s OTC Final Monograph for Topical Antifungal Drug Products.
- Greek prosecutors raid Novartis offices, disclose wide-ranging probe into bribery allegations (fiercepharma.com)
Greek officials announced Tuesday that they are investigating Novartis for bribery in the wake of local media reports raising questions about the company. It is the fourth set of bribery allegations…to go public in the past year…Greek authorities have interviewed scores of sources and raided Novartis offices in Greece, according to multiple local news outlets. Justice Minister Stavros Kontonis ordered the inquiry after "denunciations concerning bribes paid to functionaries by Novartis" appeared in the press… Greece’s investigation follows a similar probe launched in South Korea last February and corruption allegations leveled by a Turkish whistleblower…South Korean prosecutors indicted a half-dozen Novartis executives for issuing improper rebates to local doctors…last year, Novartis agreed to pay $25 million to settle an SEC investigation into bribery allegations in China…Novartis offered Chinese doctors lavish entertainment…and other inducements to boost prescriptions of its drugs in the country.
- Las Vegas doctor, 92, on trial in federal drug case (reviewjournal.com)
...Dr. Henri Wetselaar...his medical assistant (David Litwin) and a pharmacist (Jason Smith)...are accused of funneling large quantities of pills onto the streets of Las Vegas through an illegal prescription drug ring...the trial...has provided a window into the scope of the federal government’s crackdown on prescription drug abuse in Southern Nevada...One of the government’s star witnesses is a drug dealer who testified this week about an arrangement she had with Wetselaar and Litwin, who saw clients out of her home twice a week. Carolyn Allen said she would refer clients to Wetselaar, instruct them to complain about back pain, and provide them with the cash to pay for the prescription. Clients would return to her with the prescriptions...she would take the prescriptions to Lam’s Pharmacy, where Smith was the manager. She said Lam’s Pharmacy maintained an entire book dedicated only to her clients. Allen said her clients were prescribed — among other drugs — oxycodone, hydrocodone, Soma and Xanax.
- Henderson man pleads guilty in $100M health care fraud scheme (reviewjournal.com)
A Henderson man has pleaded guilty to fraudulently distributing more than $100 million worth of prescription drugs that came from the black market...Randy Crowell, 56, entered the plea last week in federal court...on one count of conspiracy to commit health care fraud. The case stretches from Utah, where Crowell’s wholesale distribution company was based, to street corners of Manhattan and the Bronx, where people sold their prescriptions to low-level collectors for $40 a bottle or more...Crowell gained about $16 million in profits…Crowell ran the scheme...as the operator and owner of a licensed wholesale distributor of prescription medications...During that time, he and co-conspirators funneled prescription medications from the black market to pharmacies nationwide...He exposed people with life-threatening illnesses to medicines they had no idea had been diverted from the normal stream of commerce, all the while defrauding health care companies and government benefit programs…
- Nevada State Board of Pharmacy – Newsletter January 2017 (bop.nv.gov)
- Changing Faces
- Bowl of Hygeia Awarded to Adam Porath, PharmD
- No Prescription Needed!
- FDA Issues Final Rule Amending List of Drug Products That May Not Be Compounded
- Selected Medication Risks to Manage in 2017
- Environmental Factors, Workflow, and Staffing Patterns – Poor Quality Lighting
- DEA to Decrease Manufacturing Amount of Opioid Controlled Substances in 2017
- New CDC Brochure Offers Pharmacists Tips for Addressing Prescription Opioid Abuse and Overdose
- FDA Requires Boxed Warnings and Patient Focused Medication Guides Indicating Serious Risks Related to Combined Use of Certain Opioid Medications and Benzodiazepines
- FDA’s Division of Drug Information Offers CE Webinars for Students and Clinicians
- FDA Approves Labeling Changes for All Prescription Testosterone Products
- Latest FDA Drug Info Rounds Training Videos Available
- Inspector's Corner
- Ghost Towns and Medicines
- McKesson in record $150 million U.S. settlement over suspicious drug orders (reuters.com)
McKesson Corp, one of the largest U.S. distributors of pharmaceutical drugs, will pay a record $150 million to resolve a U.S. investigation into whether it failed to report suspicious orders of addictive painkillers...Tuesday's deal...followed an earlier settlement with the company over similar violations in 2008...Under the settlement, San Francisco-based McKesson must on a staggered basis suspend sales of controlled substances from distribution centers in Colorado, Ohio, Michigan and Florida for several years...The Justice Department said the evidence showed that McKesson did not fully implement or adhere to its own compliance program designed after the 2008 settlement.
- Hospital Fined $510,000 After Pharmacist’s Illicit Prescription Drug Diversion (pharmacytimes.com)
A Pennsylvania hospital has agreed to pay $510,000 in fines after an inpatient pharmacist stole controlled substances. The pharmacist, Renata Dul, was found to have stolen more than 35,000 units of a controlled substance, including oxycodone….Abington Memorial Hospital disclosed the pharmacist’s actions to the DEA after detecting a discrepancy during inventory, which prompted an investigation that revealed pill count discrepancies, missing or incomplete medication inventories, and altered or missing drug records. An internal investigation pinpointed Dul’s ability to exploit a gap in the software used to track prescription medications...AMH agreed to pay a fine in response to allegations that their failures in control and practices enabled Dul to divert controlled substances for illegal, non-medical uses...
- DEA opens shop in China to help fight synthetic drug trade (hosted.ap.org)
In a sign of improving cooperation between the U.S. and China to fight the global drug trade, the Drug Enforcement Administration will open a new office there...acting administrator Chuck Rosenberg will visit Beijing, Guangzhou and Hong Kong...at the invitation of China's Ministry of Public Security...DEA maintains that China is the top source country for synthetic opioids like fentanyl and its precursors, which have been fueling the deadliest drug abuse epidemic in U.S. history. China is also emerging as a laundering destination for drug money... Beijing has taken significant steps to crack down on the production and export of synthetic drugs, even though these substances are not widely abused within China...