- Drug regulation: 27 medicines sold by top firms ‘fail’ quality tests in seven states (indianexpress.com)
In a major crackdown...the drug regulators of seven states have alleged that 27 medicines — sold by 18 major drug companies in India including Abbott India, GSK India, Sun Pharma, Cipla and Glenmark Pharma — are of "substandard" quality, citing grounds such as false labelling, wrong quantity of ingredients, discolouration, moisture formation, failing dissolution test and failing disintegration test...These include key drug brands of eight top-tier companies, which are the leaders in their respective molecule categories with a market share ranging from 47 per cent to 92 per cent. Of the 18 companies, only two said they had stopped sale of the affected drug batches and just one said the affected batch had been recalled from the market...Only eight companies responded to specific queries sent by The Indian Express on the findings of the regulators. Among the reasons they cited were: drug batches were picked up for testing from an "unofficial distributor"; no "labelling requirement" as drug batch was meant for the World Health Organisation; a batch of "counterfeit" drugs were picked up for testing; test conducted on the drug was "not necessary"; testing methodology "incorrect"; the company was doing contract manufacturing for someone else; "inappropriate storage" and "handling" in the marketplace (retailer).
- Teva says production halted at Rimsa plant at the request of Mexican regulator (fiercepharma.com)
Teva has halted production at the Rimsa plant in Mexico and idled some of the workers, laying the blame for the issues at the feet of the brothers with whom they are battling in court over the $2.3 billion buyout...Teva acknowledged today that Mexican authorities suspended manufacturing at the plant in October saying the action followed...discovery of the serious violations committed under the Espinosa brothers, Rimsa's former owners....We are working closely with the Mexican authorities in order to restore production and products to the market…Mexico’s Federal Commission for Protection against Health Risks...took its action in response to a complaint by Teva. The agency is now said to be verifying that the 140 products manufactured at the plant in Guadalajara meet quality, safety and efficacy standards. COFEPRIS expects to complete its analysis by year-end...
- DOJ turns tables on Express Scripts, demands info on pharma deals (fiercepharma.com)
Express Scripts disclosed this week that the U.S. Attorney’s offices in New York and Massachusetts had demanded information about two different issues: financial ties with pharma companies, and relationships among drugmakers, patient assistance programs and the specialty pharmacies that fill prescriptions...Specifically, the federal prosecutors in New York want information about money changing hands between Express Scripts and pharma companies. That would include rebates that drugmakers pay to win favorable reimbursement deals for their products...The newly disclosed Express Scripts probes aren’t the first DOJ demands for information about PBM-pharma relationships. In a series of financial filings in May, it became clear that the DOJ was looking for information across the industry. Johnson & Johnson, Merck & Co. and Endo International all said they were being asked for info...secrecy could be coming to an end. Politicians and patient advocates are calling for more transparency from PBMs and drugmakers alike, and obviously, the DOJ is doing the same.
- SEC charges former Puma biotech exec with $1.1 million in insider trading (statnews.com)
In the latest instance of alleged insider trading in the pharmaceutical industry, a former Puma Biotechnology executive was charged with illegally making more than $1.1 million by taking advantage of confidential information about clinical trials for a cancer drug...Robert Gadimian, who was senior director of regulatory affairs, bought and sold Puma stock after learning about favorable study results for a medicine that was being tested to treat breast cancer…This alleged episode of insider trading is only the latest instance involving the pharmaceutical industry or those working with drug makers. The issue has increasingly raised concerns in connection with clinical trial work, as well as deal-making and the drug approval process, which some fear can be distorted by such activities.
- NYPD Union Goes After Drug Prices Amid DOJ Pharma Probe (bloomberg.com)
As the generic drug industry braces for charges from a two-year U.S. Justice Department antitrust investigation, a union representing the sergeants of the New York Police Department is attempting to hit some companies with civil penalties as well...A pair of lawsuits filed by the Sergeants Benevolent Association Health & Welfare Fund against two groups of drugmakers, which include...Novartis AG’s generic drug unit,...Perrigo Co., India’s Wockhardt Ltd. and Taro Pharmaceutical Industries Ltd., allege the companies colluded to raise prices on two dermatological creams as much as 1,000 percent…U.S. pharma sector is now facing sharp scrutiny on pricing, including a sweeping Justice Department probe. That antitrust investigation spanning companies from around the world is examining whether some executives agreed with one another to raise prices on generic medicines in the U.S...
- The Cost of Counterfeits (pharmtech.com)
The proliferation of counterfeit medicines is nothing new to pharma; however, the scale of the problem seems to be escalating, especially with the Internet providing an easy means for fraudsters to dispense their fakes. Counterfeiting has a devastating impact on public health and the economy. Not only are consumers paying for products of inferior quality, but their wellbeing is also put at risk. For genuine drugmakers, profits are diluted, but the repercussions extend beyond that…The European Union Intellectual Property Office reported...that the pharmaceutical industry is stripped of approximately €10 billion ($ xxxxx) of revenue every year because of counterfeit medicines...the lost sales translate into 37,700 jobs lost across the pharmaceutical sector in the EU as a result of legitimate manufacturers and distributors employing fewer people than they would do had this problem not existed…With serialization and track-and trace legislations being rolled out over the next few years, pharma is doing its part to secure its supply chain. The problem will be an ongoing challenge for the industry, but with advances in technology, it will become easier to detect the fakes in the near future...
- DEA and Partners Hold Prescription Drug Take Back Day Saturday (dea.gov)
DEA reprises this weekend one of its most popular community programs: National Prescription Drug Take Back Day. On Saturday October 22 between 10 a.m. and 2 p.m., the public can dispose of their unused, unwanted prescription medications at one of 4,700 collection sites nationwide, operated by 3,800 local law enforcement agencies and other community partners. The service is free of charge, no questions asked.
- With Legal Pot Comes a Problem: How Do We Weed Out Impaired Drivers? (realclearhealth.com)
On Nov. 8 voters in California, Maine, Massachusetts and Nevada approved ballot measures to legalize recreational cannabis. It is now legal in a total of eight states. And this creates potential problems for road safety. How do we determine who’s impaired and who’s not?...The effects of alcohol vary based on a person’s size and weight, metabolism rate, related food intake and the type and amount of beverage consumed. Even so, alcohol consumption produces fairly straightforward results: The more you drink, the worse you drive. Factors like body size and drinking experience can shift the correlation slightly, but the relationship is still pretty linear, enough to be able to confidently develop a blood alcohol content scale for legally determining drunk driving. Not so with marijuana...Second to alcohol marijuana is the drug most frequently found in drivers involved in crashes...But how do you know when you’re too stoned to drive? How can police tell?...the Center for Medicinal Cannabis Research at UC San Diego...received a...$1.8 million grant from the state of California to gather data about dosages, time and what it takes to impair driving ability...then create a viable roadside sobriety test for cannabis...
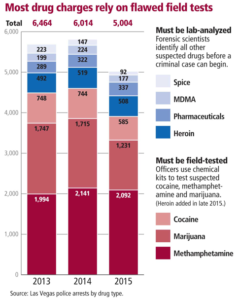
- Defense attorneys want to challenge Las Vegas police use of faulty drug tests (reviewjournal.com)SPECIAL INVESTIGATION: Las Vegas drug convictions rely on faulty police field tests (reviewjournal.com)
A prominent organization of defense lawyers in Las Vegas this week formed a committee to explore ways of challenging local law enforcement’s methods for gaining drug convictions...The committee, set up by the Nevada Attorneys for Criminal Justice, will look at the use of what are known as chemical field tests, inexpensive kits used by police and prosecutors to make drug arrests and gain guilty pleas. Officers typically drop suspicious materials into a chemical pouch and look for telltale shifts in color ostensibly meant to indicate the possible presence of illegal drugs. The tests are often the only evidence used to win convictions...The Las Vegas Metropolitan Police Department crime lab had submitted a formal report detailing the shortcomings of the tests to federal authorities in 2014, and yet to this day the lab still endorses the use of the tests in criminal prosecutions...
- To stop FDA inspector, workers at Japanese drug maker formed a human barricade (statnews.com)
Someone at Nippon Fine Chemical must have been very nervous when an investigator from the US Food and Drug Administration arrived last year...In an unusual display of chutzpah, the drug and ingredients maker refused to allow the investigator to inspect its quality control laboratory at its facility in Hyogo, Japan. Employees literally formed a "shoulder-to-shoulder" barricade to prevent the FDA employee from entering…the FDA investigator reviewed customer complaints that Nippon drugs contained glass, hair, cardboard, metal — and even a black spider. But Nippon employees refused to provide the investigator with copies of documents that detailed customer complaints. The FDA letter noted that Nippon uses the same equipment and processes for drugs sold in the Japanese and US markets...As a result of these foibles, the FDA declared Nippon products to be adulterated and issued an import alert two months ago, an action that means Nippon products can be detained from entering the US...Nippon could not be reached for comment...