- FDA slaps Pfizer’s Hospira unit for ‘misleading’ YouTube ad (fiercepharma.com)
In the first enforcement action from the FDA's marketing police this year, the Office of Prescription Drug Promotion put Hospira in the hot seat over a YouTube video for its sedative Precedex (dexmedetomidine)...The OPDP sent an untitled letter dated Jan. 14 to the Pfizer-owned company, charging the video "omits risks and material facts" about the drug. The agency also rebuked Hospira for publishing the promotional video without submitting it to the OPDP for review...The letter orders Hospira to "cease violating the FD&C Act, as described," and submit a written response before Jan. 29. The response should include a plan for "discontinuing use of such violative materials," the letter states.
- Complaint alleges McKesson shipped nearly 100 million doses of highly addictive RX drugs to WV, fueled drug epidemic (wvillustrated.com)Morrisey files suit against nation’s largest drug distributor (wvgazettemail.com)
Prescription drug distributor McKesson Corporation is the target of a complaint alleging it fueled West Virginia's prescription drug addiction problem by "failing to identify, detect, report and help stop the flood of suspicious drug orders into the state," Attorney General Patrick Morrisey said…the...complaint…alleges McKesson flooded West Virginia with highly addictive prescription medications, delivering roughly 99.5 million doses of hydrocodone and oxycodone…McKesson…"made no efforts to determine whether the volume of prescription pain killers it was shipping ...was excessive and whether any of the orders it filled qualified as suspicious orders, which should have been refused."…Sales agents and managers received commissions and bonuses based on sales numbers, and made "little to no effort to visit pharmacies" to ensure shipments weren't being diverted to illegal use…"In the near future, the office will seek to join this case with the ongoing matter in Boone County involving 12 other drug wholesaler defendants," he said in a prepared statement…in order to coordinate the Amerisource and McKesson cases and to ensure adequate resources are available to prosecute the McKesson case, the state has awarded an outside counsel appointment...Morrisey also announced Jan. 8 he will be handing off the management of both the Amerisource and McKesson cases to...Anthony Martin and...Vaughn Sizemore and will voluntarily step aside, going further than the rules require. Morrisey has had ties to Cardinal Health, one of the nation's largest drug distributors.
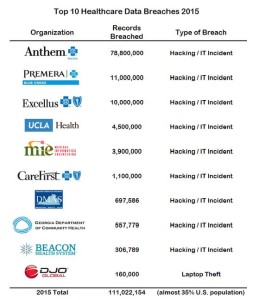
- Data Breaches In Healthcare Totaled Over 112 Million Records In 2015 (forbes.com)Top Pharmacy Chains Revealed as Repeat HIPAA Violators (pharmacytimes.com)
Healthcare’s “wall-of-shame” for 2015 officially ends tonight at midnight. It’s not really a “wall,” it’s just a website, but it’s the online mechanism for the Office of Civil Rights under Health and Human Services to publish data breaches as reported to them and required by HIPAA. The numbers this year are just staggering...According to OCR, there were 253 healthcare breaches that affected 500 individuals or more with a combined loss of over 112 million records...The Top 10 data breaches alone accounted for just over 111 million records that were lost, stolen or inappropriately disclosed...A recent data breach study estimates that breaches cost the healthcare industry about $5.6 billion annually. As healthcare moves toward connected care, the amount of data exchanged between organizations will only grow. So what does this mean? It means that in 2016, we’re going to see a huge movement towards encryption in hospitals and other healthcare facilities in order to protect EHRs and other vulnerable PHI...Healthcare IT security will continue to fall further and further behind the rest of the industry verticals despite the increase in spending on technology and human resources. The industry is focusing on functionality for patient care and security is an afterthought. Many organizations are also overly dependent on antiquated hardware and software...I wish we could look back on 2015 as the year that healthcare took data security and patient privacy more seriously...In a data-driven world, medical information is just too lucrative and too easy to steal at scale. As long as that’s the case...we should reasonably expect more of the same for 2016.
- KaloBios, formerly led by Shkreli, files for bankruptcy (finance.yahoo.com)
After Shkreli arrest, 2 drugmakers are upended, 1 seeking bankruptcy protection...KaloBios, the troubled drugmaker taken over by Martin Shkreli last month, is seeking bankruptcy protection less than two weeks after his arrest for securities fraud...It is the second pharmaceutical company with ties to the former hedge fund manager now in turmoil following his indictment on charges unrelated to his involvement with them, though the drugmakers are not lacking for problems of their own...Turing Pharmaceuticals Inc., is cutting jobs and seeking a new CEO after Shkreli resigned the position because of his arrest...Trading in KaloBios shares has been suspended for two weeks and it was notified one week ago that it would be delisted from Nasdaq because of Shkreli's arrest, as well as the arrest of the company's outside counsel...In a Chapter 11 filing...with the U.S. bankruptcy court...the company listed assets and liabilities in the range of $1 million to $10 million...KaloBios' largest creditors include the University of Miami, Ernst & Young and Lonza Sales Ltd.
- 5 Accused of Stealing Drug Secrets From GlaxoSmithKline (nytimes.com)
Federal prosecutors in Philadelphia said on Wednesday that they had indicted five people, including two research scientists, on charges of stealing trade secrets about drugs to treat cancer and other diseases from GlaxoSmithKline...the two scientists, Yu Xue and Lucy Xi, worked at Glaxo’s research facility in Upper Merion, Pa., and emailed and downloaded confidential data about a dozen or more company products to associates who planned to sell and market the trade secrets through a company they set up in China, called Renopharma...to conceal their crime, Ms. Xue and two other associates, Tao Li and Yan Mei, agreed to put the proceeds in the name of Ms. Xue’s twin sister...who was also charged. Ms. Xi worked with Ms. Xue at Glaxo and was married to Mr. Mei...the defendants boasted that their company, based in Nanjing, had received some financial support and free laboratory space from the government and that its ultimate goal was to develop its own antibody drugs.
- “Pill Mill” Doctor Pleads Guilty to Drug Distribution and Money Laundering Charges (dea.gov)
Drug Enforcement Administration...announced...Dr. Francisco Huidor-Figureoa, 48, a physician...Montgomery, Alabama, pleaded guilty...to one count of conspiring to distribute oxycodone and one count of conspiring to commit money laundering...During the plea hearing...Figureoa admitted that he worked...a “pill mill.”...was the sole physician employed by the EMED Medical Management Corporation...in Opelika, Alabama...Figureoa sold oxycodone to pill dealers, based on a fraudulent prescription...knew that the recipients of these illegal pills did not need the medicine and that the recipients intended to either abuse the pills or sell the pills to others who would abuse them...Figueroa faces up to 20 years’ imprisonment on each count...Additionally, on the drug distribution conspiracy count...faces a maximum fine of $1,000,000. On the money laundering conspiracy count, the maximum fine...could be...$500,000, or twice the value of the property involved in the transaction, whichever is greater.
- Your health records are supposed to be private. They aren’t. (washingtonpost.com)
The federal law that protects health information is violated often and easily, and it's hardly ever enforced...After spending the past year reporting on loopholes and lax enforcement of the Health Insurance Portability and Accountability Act, the federal patient-privacy law known as HIPAA, I’ve come to realize that it’s not just celebrity patients who are at risk. We all are...I’ve talked to hundreds of people who say their medical records were hacked, snooped in, shared or stolen...In each story, a common theme emerged: HIPAA wasn’t working the way we expect. And the agency charged with enforcing it, the HHS office for civil rights, wasn’t taking aggressive action against those who violated the law...We all know HIPAA... It’s what requires us to stand behind a line, away from other customers, at the pharmacy counter or when checking in at the doctor’s office...It is used to scare health-care workers, telling them that if they improperly disclose others’ information, they could pay a steep fine or even go to jail...But in reality, it is a toothless tiger...And even though the civil rights office can impose large fines, it rarely does: It received nearly 18,000 complaints in 2014 but took only six formal actions that year. A recent report from the HHS inspector general said the office wasn’t keeping track of repeat offenders, much less doing anything about them...Making matters worse, HIPAA does not allow patients to sue health providers for damages if they violate the law. So if the federal government doesn’t enforce the law, there are often no consequences for breaking it...Moreover, the government needs to write regulations to implement provisions of a 2009 law that would give patients whose privacy has been violated a share of the money HHS recovers. Finally, the government has yet to submit to Congress a report due in 2010 with recommendations for how to deal with the privacy of health information not covered by HIPAA.
- Compounding Pharmacy Forced to Stop Production Due to Insanitary Conditions (specialtypharmacytimes.com)Federal judge enters consent decree against Downing Labs (fda.gov)FDA sues to stop a wayward drug compounder (statnews.com)
Compounding pharmacy Downing Labs LLC (formerly known as NuVision Pharmacy), its co-owners, and its pharmacist-in-charge have been issued a consent decree of permanent injunction...The Texas-based company is allegedly in violation of current good manufacturing practice requirements under the Federal Food, Drug, and Cosmetic Act...Downing Labs is accused of manufacturing and distributing adulterated drugs that were made in insanitary conditions, meaning they were bad enough to endanger public health...“Despite multiple warnings to the company, Downing Labs continued to manufacture injectable drugs under insanitary conditions, putting the health and safety of patients at risk,”...“The FDA pursued appropriate and aggressive action to protect the public health.”...Downing Labs said it has worked "collaboratively and cooperatively” with the FDA to reach an agreement that will enable it to resume the production of compounded sterile medication...also noted that, as part of the consent decree, it voluntarily agreed to participate in a regular program of testing, audit, and inspection “to ensure it is achieving and exceeding its quality goals.”
- China continues to fine-tune drug, food safety procedures (fiercepharmaasia.com)
The government of China continues to refine its legal powers to deal with drug safety issues, according to a release from the country's Supreme Court that says investigations will be streamlined to identify administrative cases that potentially involve major criminal breaches...The new measures are designed to "facilitate coordination between administrative and judicial organs in handling food and drug safety cases," according to a report from the Shanghai Daily...The China Food and Drug Administration and the Ministry of Public Security, the Supreme People's Court, the Supreme People's Procuratorate and the executive office of the food safety commission under the State Council, China's cabinet, said the measures will "streamline" standards and procedures and will include the possibility of suspected drug cases being transferred from administrative bodies such as the CFDA to police...These latest moves are an attempt by Chinese officials to bring trust to the "Made in China" label because most Chinese prefer foreign-made drugs which they believe are higher quality. Several Chinese and Indian companies in recent months have been slammed by regulatory authorities in the United States and Europe over lapses in good manufacturing practices and outright fraud in many cases where test results were falsified.
- Cadila Healthcare shares plunge after FDA warns of violations (reuters.com)
Cadila Healthcare Ltd (Zydus Cadila)has received a U.S. Food and Drug Administration warning letter for violating manufacturing standards at two of its production facilities, the latest in a series of Indian companies to face such action....The warning letter cites issues with Cadila's plants in Gujarat, including at the Moraiya facility, which makes up about 60 percent of the company's total sales in the United States, its largest market...Dozens of Indian drug plants have faced warnings and bans in recent years, as the FDA improved inspections of foreign facilities. More than 40 percent of the generic and over the counter medicines available in the United States comes from Indian facilities such as Cadila's Moraiya plant...Cadila Managing Director Pankaj Patel told analysts...the FDA, during an inspection of the Moraiya plant...found deficiencies with the way the company investigated market complaints about a medicine made there...The company is working on a response to the warning letter and will then ask the FDA to reinspect both facilities...It has 15 days to respond to the FDA, as per standard procedures, after which the FDA will decide its response including whether to impose an import ban.