- House committees introduce new healthcare price transparency legislation (fiercehealthcare.com)
A trio of House committees unveiled new legislation...to lower costs and increase transparency for patients...The Lower Costs, More Transparency Act includes provisions from the House Energy and Commerce Committee, the Ways and Means Committee and the Education and the Workforce Committee and is designed to help patients be more informed when making healthcare decisions regarding cost of care, treatment and services...It requires hospitals, payers, labs, imaging providers and ambulatory surgical centers to list prices they will charge patients through machine-readable files and mandates insurers and pharmacy benefit managers disclose drug rebates and discounts, according to a news release...READ MORE
- Bipartisan caucus in US Congress looks to boost domestic drug manufacturing (fiercepharma.com)
...the Domestic Pharmaceutical Manufacturing Caucus for the 118th Congress...will focus on domestic production of finished drugs and active pharmaceutical ingredients...Members of the caucus said they will look to advance legislation that incentivizes more domestic production for essential medicines as part of an effort to reduce U.S. reliance on foreign countries. The lawmakers also aim to head off potential supply chain disruptions and ensure a steady supply of pharmaceuticals in the event of public health emergencies...READ MORE
- Government spending bill would tighten FDA oversight of accelerated drug approvals (biopharmadive.com)
The Food and Drug Administration could require drugmakers seeking speedy approvals of new treatments to begin confirmatory testing before gaining the agency’s clearance under legislation before Congress...the legislation would also expedite the process for withdrawing conditionally approved drugs...Contained in a massive, year-end federal spending bill, the provisions instruct the FDA to convene a seven-member council of agency officials to develop consistent policies and practices around accelerated approvals...READ MORE
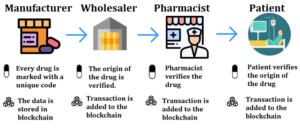
- How Pharmacies Can Prep Now for the 2023 DSCSA Requirements (nabp.pharmacy)Drug Supply Chain Security Act (DSCSA) (fda.gov)
When Food and Drug Administration enacted the Drug Supply Chain Security Act in 2013, it set a 10-year timeline for full implementation. Well, believe it or not, we are just a little more than a year away from that November 2023 deadline...A significant portion of this last milestone requires the entire supply chain to become interoperable using secure and electronic means. One requirement that is included in Title II of the Act calls for product tracing at the package level, which had not been explored widely by the industry until recently. Our DSCSA State Regulator Pilot project tested a system for sharing this information between regulators and trading partners, including distributors, manufacturers, and others...READ MORE
- Facing a nursing ‘crisis,’ Nevada lawmakers invested $20 million for nursing schools (thenevadaindependent.com)
Earlier this year, Nevada lawmakers unanimously passed SB375, which allocates $20 million over the next two fiscal years to increase the number of nursing faculty and graduates at seven state nursing programs — an effort to address the state’s troubling nursing shortage..., some school leaders cautioned that main drivers of the nursing shortage — such as pay for faculty and clinical nurses and burnout among nurses — remain unaddressed...It’s also difficult to hire nursing faculty because of the lower pay and higher education requirements in academia compared to clinical settings...READ MORE
- Walgreens won’t sell abortion pills in GOP states after legal threats from state officials (fiercehealthcare.com)
Walgreens will not dispense abortion pills in nearly two dozen states after legal threats from GOP lawmakers, the retail pharmacy chain confirmed...The Walgreens decision stems from a letter written by nearly two dozen Republican state attorneys general at the beginning of February that threatened legal action if the company began distributing mifepristone in their states...Walgreens...spokesperson saying “we are not distributing mifepristone at this time. We intend to be a certified pharmacy and will distribute mifepristone only in those jurisdictions where it is legal and operationally feasible.”...READ MORE
- Inflation Reduction Act and Its Impact on Pharmaceutical Pricing and Investment Decisions (drugtopics.com)
The reference to “maximum fair price” in the act bodes poorly for manufacturers and suggests more of a take-it-or-leave-it situation rather than a negotiation where clinical evidence would be the prevailing factor in determining price...Now that the dust has settled over enactment of the Inflation Reduction Act, leaders across the industry should be taking stock and assessing how the law will impact their product portfolios and the bottom line. Although several open questions remain, pharmaceutical manufacturers can take actions to best position their organizations to either benefit from — or mitigate repercussions — of the new law. Specifically, executives should lay out strategies for addressing revenue optimization, evidence development planning and portfolio optimization...READ MORE
- Don’t let drug companies run Nevada’s health care industry (thenevadaindependent.com)Medication prices could be capped under proposed Nevada bill (reviewjournal.com)
Members of both political parties agree that keeping life-saving prescription drug prices reasonable saves lives; however, there is an ongoing debate about the best way to accomplish this goal...For many Democrats, government-imposed price controls are the solution. This past legislative session, they passed Assembly Bill 250, which would prevent the major drug manufacturers from inflating the cost of our prescription drugs by having the government set these products’ prices...Republicans, on the other hand, argue that promoting marketplace competition is a more effective solution than more government. That’s why Gov. Joe Lombardo rightly vetoed this bill...Regardless of where one falls on the political spectrum, everyone in this state should agree that regulating away the private market entities that help restrain the drug companies’ ability to rig prices higher shouldn’t be the answer. And yet, that’s exactly what the drugmakers are telling them to do...READ MORE
- States Move to Ban Accumulators (drugtopics.com)The State of Employers’ Pharmacy Benefits: A Review of 2022 and the 2023 Outlook for Copay Programs (drugchannels.net)How Copay Accumulators and Maximizers Have Changed Payers’ View of Copay Support (drugchannels.net)
Sixteen states have banned a pharmacy benefit management practice that involves not counting the value of drug copay assistance from manufacturers toward patient deductibles...Drugmakers use copay assistance programs to shield patients from out-of-pocket expenses — and build market share for their products in the process. But pharmacy benefit managers have cried foul, saying the copay programs undercut formularies and wind up increasing the use of expensive drugs that are not any better than less expensive ones. They have pushed back with “copay adjustment programs,” especially “copay accumulators,” which are designed to blunt the effect of the copay assistance programs by not counting their value toward patient deductibles...READ MORE
- Pharma earnings outline drug law’s looming impact on sales, development (biopharmadive.com)
While many companies are still unsure of the law’s effects, some have begun to warn investors about the likelihood of lower sales and reduced profitability...Biotechnology and pharmaceutical companies are still parsing the U.S. drug pricing law enacted in August, but recently have begun to warn investors of looming impacts to their development plans and to future sales...In regulatory filings and earnings calls over the past few weeks, major drugmakers described significant uncertainty over how the law will be implemented and how it might directly affect their business. But some have been more specific, with Merck & Co. and Amgen noting they expect it to impact future sales. And a few, including Eli Lilly, Alnylam Pharmaceuticals and Alkermes, have cited the law specifically in decisions to cut back or reshape their drug research...READ MORE