- New Nevada law aims to tackle opioid epidemic (reviewjournal.com)
Doctors have additional protocols to consider when writing and maintaining opioid prescriptions under a new law that took effect on New Year’s Day...The Prescription Drug Abuse Prevention Act, passed by the 2017 Legislature, outlines safeguards for doctors before they prescribe controlled substances to treat pain and increases requirements necessary to continue a prescription after one month, three months and a year...The additional paperwork is meant to curb the state’s opioid overdose problem and track down doctors who overprescribe...“It just provides a platform by which the provider can really have an in-depth discussion with the patient as to whether the use of a controlled substance is truly necessary, or whether there are alternatives, ” said Daniel Burkhead, a pain management specialist in Las Vegas...The guidelines require every doctor to perform a patient risk assessment before prescribing a controlled substance to treat pain...Nevada is among 17 states that have enacted legislation limiting the number of days of an initial opioid prescription or capping prescription strength...
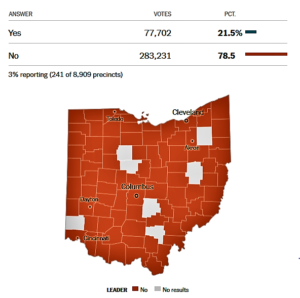
- Ohio Ballot Issue 2: Cap State Agency Drug Costs (nytimes.com)Handing pharma a win, Ohio voters overwhelmingly reject drug pricing measure (fiercepharma.com)
An initiative on Tuesday’s ballot in Ohio is aimed at reducing the cost of prescription drugs in the state. The measure would cap the price of prescription drugs purchased by the state government, including Medicaid...The measure drew strong resistance from drug makers, which spent more than $49 million to try to kill it, and the industry is not accustomed to losing political fights. Last year, it successfully killed a measure in California that was similar to the one in Ohio, but only after spending more than $100 million to do so.
- California deals pharma a double whammy with signing of copay coupon bill (fiercepharma.com)California Takes On Drug Pricing: Real Progress Or Illusion? (healthaffairs.org)
Gov. Jerry Brown signed California's AB-265, which will limit the use of copay coupons and other discounting strategies for branded prescription drugs when a cheaper generic is available. Brown authorized that bill on the same day he signed SB-17, a separate piece of legislation which will force drugmakers to give warning of and explain price hikes...Exceptions to the copay coupon law include cases in which patients complete step therapy or receive prior authorization for the branded drug...the bills represent two significant regulatory steps on drug pricing...they are a setback for the drug industry in California. But other states could follow the model as many around the country look to take pharmaceutical prices into their own hands...PhRMA,...opposed both proposals...concerned with AB-265 because it doesn't ensure that patients who need a branded med over a generic will be able to get that drug...When patients receive short-term co-pay assistance for expensive drugs, they may be insulated from price hikes, but insurance companies, the government and employers still bear the burden of these excessive prices...these costs are eventually passed on to consumers in the form of higher premiums...
- Nevada Legislature looks to defend law against drug companies (reviewjournal.com)
The Nevada Legislature has filed a motion to intervene as a defendant in a federal lawsuit that challenges a state law that requires disclosure of how insulin is priced...Pharmaceutical Research and Manufacturers of America and the Biotechnology Innovation Organization say the Nevada law passed this year is unconstitutional. The groups maintain the legislation improperly interferes with federal patent law because the disclosure requirements it imposes penalize pricing decisions that are consistent with the patent rights granted by Congress...Gov. Brian Sandoval and Department of Health and Human Services Director Richard Whitley are named as defendants in the lawsuit. The Legislature on Tuesday filed its motion to defend the law.
- Officials explain bill to curb opioid overprescription in Nevada (reviewjournal.com)
Nevada health officials on Tuesday said a bill aimed at curtailing opioid overprescription will keep decision-making in the hands of physicians, not lawmakers...The provisions of Assembly Bill 474, which the Legislature passed into law this year and takes effect on New Year’s Day, were explained to about 200 physicians Tuesday evening at a forum at Las Vegas City Hall...Daniel Burkhead, a physician at the Innovative Pain Care Center in Las Vegas, said the law asks doctors to exercise caution...The law mandates that doctors conduct mental health evaluations before issuing first-time opioid prescriptions, which will be limited to 14 days. The law also mandates that doctors register with the state’s Prescription Drug Monitoring Program...“(Lawmakers) did not want to take power out of the hands of physicians,” Burkhead said. “The law wants you to think, before writing that prescription, if this patient provides a high risk of that medication being diverted or abused or misused.”
- The Unhealthy Politics of Pork: How It Increases Your Medical Costs (nytimes.com)
No industry in America spends more on lobbying than health care...In 2016, the health care industry spent half a billion dollars on lobbying, with pharmaceutical companies, hospitals and health professionals making the largest contributions...Closely related to industry lobbying is the political maneuvering that congressional leaders use in an effort to pass legislation — specifically, targeted provisions known as earmarks, “sweeteners” or pork barrel spending...In 2010, Democrats hoping to secure votes from reluctant rural state senators added the “Frontier States” provision to the A.C.A., which increased Medicare payments to five states with low population densities...We all know earmarks and lobbying influence policymakers and policy. In health care, this has critical implications: who gets care, how much they get, how we pay for it. But there’s little hard data on exactly who benefits and how large the effects can be. A new study illuminates the ways these political dynamics can change congressional and hospital behavior — and how they can increase health care costs for the rest of us...America’s increasingly burdensome health care spending has many roots: new technologies, high drug prices, fragmented care, administrative expenses and the like. But lobbying and political maneuvering can increase costs, too — without clear benefits for patients, communities or society at large...Often these costs are borne by all of us, while the benefits — if any — go to a favored few. Excess medical spending, then, is driven not only by inefficiencies in our health system, but also by those in our political system. Our solutions, it seems, must confront that uncomfortable reality.
- Nevada explores options as Children’s Health Insurance Program expires (reviewjournal.com)
Nevada officials are exploring alternative avenues for insuring more than 26,300 children in the state covered under the Children’s Health Insurance Program if Congress doesn’t reauthorize the program, which is set to expire Saturday...Congress is expected to reauthorize the CHIP program through a bipartisan bill, though that could be weeks away. And with the turmoil in Congress lately, state officials are taking no chances...The Department of Health Care Financing and Policy is working with state partners to identify alternative sources of funding for the program if it is not reauthorized, deputy administrator Cody Phinney said ...“We would have to find other funding sources and we’d have to look at our options for limiting the services that are available, but our first role is to maintain those services,” Phinney said...the federal government pays nearly the entire annual $43 million cost of the program in Nevada...The state has ‘reserve funding’ to operate the health-care program for the next few months. However if Congress does not quickly reauthorize CHIP, states like Nevada will need to either send notices of termination to program beneficiaries or develop alternative funding...
- DEA cracking down on fake fentanyl traffickers (statnews.com)
The U.S. Drug Enforcement Administration wants to make it easier for federal prosecutors to go after people who peddle illicit versions of the deadly opioid fentanyl that are fueling the nation’s drug abuse crisis...The agency said Thursday it intends to classify drugs that are chemically similar to fentanyl as illegal controlled substances, letting prosecutors avoid the hurdles they often face in bringing charges in such cases...The move aims to stop the flow of fentanyl variants into the U.S. as the opioid abuse crisis rages...The new classification, which would last two years, lets prosecutors bring charges against fake-fentanyl traffickers under the federal Controlled Substances Act. Prosecutors have been able to use the Analogue Act for cases involving variants, but that requires them to call chemists and other experts to prove that, while molecularly different, the drugs are just as potent and dangerous as fentanyl in its true form. Prosecutors often complain of being stymied by the additional hurdles that delay cases and sometimes lead to charges being dropped...Law enforcement officials are frustrated by chemists who frequently alter the chemical compounds in their drugs to create substances that are not expressly illegal. Justice Department and DEA officials say they play a constant game of “whack-a-mole” to keep up with the changes.
- Thanks, California! SB17 Will Trigger Massive Speculative Buying, Windfall Pharmacy Profits, and Supply Chain Disruption (drugchannels.net)
California governor Jerry Brown has just signed SB-17 – Drug Price Transparency into state law...This law is truly nutty. It won’t accomplish much of what it purports to do...Pharmacies and healthcare providers will become the primary beneficiaries of SB17’s requirement that pharmaceutical manufacturers provide advance notice of WAC price increases. Payers and patients will see limited gains. The state of California will see no appreciable benefit...This unexpected result will occur because the revenues and profits of pharmacies are linked directly to brand-name list prices. By providing advance notice of a price increases, pharmacies will have enormous incentives to purchase extra inventory and earn windfall profits. These profits do not have to be shared with third-party payers or patients...SB17 should encourage manufacturers to begin exploring direct inventory management relationships with key pharmacies to minimize speculation and the accompanying risks of drug shortages and diversion. It will be tough to negotiate these agreements, because pharmacies will see the lure of big profits...speculative purchasing will wreak havoc with pharmaceutical supply chains. What were California’s legislators thinking? Do they have any idea how little California will benefit from advance notice? What will happen to the excess inventory and higher pharmacy profits generated by California’s new law?...Don’t ask the people who wrote the bill. They seem completely oblivious to SB17’s actual impact...
- NCPA-commissioned study: DIR fees could cost CMS $3.4B in next 10 years (drugstorenews.com)
Retroactive pharmacy payment reductions, a portion of direct and indirect remuneration fees, could cost the federal government $3.4 billion between 2018 and 2027. That’s according to a new study from Wakely Consulting...This...study is vitally important in showing that DIR legislation will actually save taxpayers $3.4 billion over 10 years without subtracting any benefits seniors currently receive...For pharmacies, banning these after-the-fact fees is the fair way to achieve predictability in the reimbursement for the medications they buy and dispense...The report evaluates the impact of the Improving Improving Transparency and Accuracy in Medicare Part D Drug Spending Act, which has been introduced in both the Senate and House of Representatives and prohibits retroactive pharmacy payment reductions as claims without any defect, impropriety or fraud in Medicare Part D...The legislation is part of NCPA’s efforts to end DIR Fees, which make up a small amount of overall DIR within Medicare Part D, the majority of which is made up of manufacturer rebates