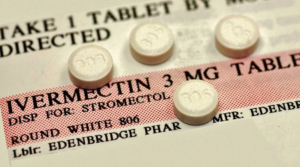
- GOP bills aimed at unproven treatments for COVID-19 (apnews.com)
Doctors and hospital leaders are pushing back against a package of Republican bills that seek to bar health care providers from withholding unproven treatments for COVID-19...The three bills released this week would prevent health systems and medical credentialing boards from disciplining doctors for ordering or advocating for therapies or medicine for patients that go against medical opinions held by their employers or regulators...
READ MORE - Few Rx supply chain stakeholders prepared to share DSCSA-required transaction data (chaindrugreview.com)
Results from a new HDA Research Foundation survey indicate that the pharmaceutical supply chain is entering a critical phase in achieving the transaction data connections required to comply with the Drug Supply Chain Security Act in 2023...The DSCSA requires transaction data with product identifiers to be provided with physical product on November 27, 2023...“many healthcare supply chain trading partners are realizing there is work to be done to establish proper business-to-business connections; ensure data are formatted, transmitted and received successfully; that processes for troubleshooting are created; and that products in inventory have the right data attached to them for shipping after November 27,” said Perry Fri...COO of the HDA Research Foundation. “The Foundation’s survey shows...what might be leading to slow implementation rates across the supply chain.”...READ MORE
- New law will allow pharmacists to administer HIV prevention medication without prescription (thenevadaindependent.com)
Nevada will become one of the first states to allow pharmacists to prescribe human immunodeficiency virus prevention drugs to patients at risk of contracting the virus, as the state works to combat one of the highest rates of HIV diagnoses in the country...A bill signed by Gov. Steve Sisolak on June 6 authorizes pharmacists with sufficient liability coverage to prescribe, dispense and administer HIV prevention drugs — including post-exposure prophylaxis to people who may have come into contact with HIV and pre-exposure prophylaxis for people at risk — without a prescription from a practitioner starting as early as Oct. 1, in accordance with protocols to be developed by the State Board of Pharmacy over the next several months...READ MORE
- Industry-heavy Patient Protection Commission could get significant membership overhaul (thenevadaindependent.com)
When Gov. Steve Sisolak proposed establishing a Patient Protection Commission to conduct a top-to-bottom review of Nevada’s health care system, he told industry representatives that his goal was compromise — and that those not working toward that goal could lose their seats at the table...Under a bill Sisolak put forward and the Legislature approved in 2019, the commission was established as an industry-heavy body, with a few patient and general public representatives added in, that would come together to address pressing health care issues in the state — in the vein of an industry working group that had successfully compromised on surprise emergency room billing legislation earlier that year...READ MORE
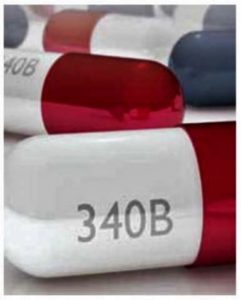
- PhRMA Litigation Challenging Constitutionality of Provisions in Arkansas Act 1103 (phrma.org)
...the Pharmaceutical Research and Manufacturers of America filed a complaint in the U.S. District Court for the Eastern District of Arkansas challenging provisions in Arkansas Act 1103 that seek to require manufacturers to provide federal 340B program pricing to Arkansas-based pharmacies contrary to federal statute...PhRMA...General Counsel James C. Stansel stated:“The 340B pricing mandate provisions of Arkansas Act 1103 violate both the Supremacy and Commerce clauses of the U.S. Constitution because they impermissibly clash with the statutory requirements of the federal 340B program. This misguided state mandate places requirements on manufacturers that directly conflict with the federal 340B statute and attempts to regulate commercial transactions that occur outside of Arkansas...READ MORE
- Part 1: Medicare Part B Provider Status for Pharmacists – Episode 1 (drugtopics.com)Part 2: Medicare Part B Provider Status for Pharmacists (drugtopics.com)
In an interview with Drug Topics®, Ken Perez, MBA, vice president of Healthcare Policy and Government Affairs at Omnicell, explained the crucial points of Medicare Part B provider status for pharmacists, which would allow pharmacists in underserved communities to be reimbursed for certain primary care services that they already provide in commercially ensured populations...READ MORE
- Johnson & Johnson among companies excluding Colorado residents from remote job openings after new state law (fiercepharma.com)90 Companies are avoiding hiring in Colorado (coloradoexcluded.com)
A senior manager in operations at Johnson & Johnson. A sales specialist at McKesson. A manager for international tax planning at Cardinal Health...What do these recent postings for remote jobs have in common?...All specify that those who live in Colorado need not apply...The issue, it seems, is Colorado’s Equal Pay for Equal Work Act, which requires companies to disclose the pay range for job openings. The goal of the new law, which went into effect on Jan. 1, is to narrow gender wage disparities by in part forcing salary transparency. Instead, companies that prefer not to disclose pay are barring remote workers from the state...READ MORE
- Community pharmacy makes goal line push to eliminate PBM spread pricing (chaindrugreview.com)
The National Community Pharmacists Association is using social media, digital advertising, and a grassroots effort to push congressional budget makers to include a provision eliminating pharmacy benefit manager spread pricing under the Medicaid program and reimburse pharmacies in a fairer and more transparent manner...“PBM spread pricing costs federal and state taxpayers hundreds of millions of dollars every year. It does nothing to reduce the cost of drugs for Medicaid patients, and it drives local pharmacies out of business,” said NCPA CEO B. Douglas Hoey...Spread pricing is what happens when pharmacy benefit managers, known as PBMs, charge insurance plans like Medicaid one price for prescription medications, reimburse pharmacies that dispense them a much lower price, and then keep a big chunk of the difference for themselves...READ MORE
- Senate takes aim at pharma’s patent schemes, pay-for-delay deals in renewed drug pricing crackdown (fiercepharma.com)
...groups lobbying for lower prescription drug prices called on Congress to enact long-awaited reforms and stressed the urgency of the movement...Answering the call...the Senate Judiciary Committee, which voted unanimously to advance four pieces of legislation which would help rein in the cost of prescription drugs...The new laws take particular aim at the tactics used by drug companies to extend patent protections and stifle competition from cheaper generic and biosimilar drugs. The legislation, which would enhance the Federal Trade Commission's ability to initiate enforcement actions against drug companies, now moves to the Senate floor for a vote...READ MORE
- Lawmakers aim to move ‘very quickly’ to pass bill mandating electronic prior authorization for MA plans (fiercehealthcare.com)
Lawmakers behind the Improving Seniors’ Timely Access to Care Act...not only requires electronic prior authorization for MA plans, but also requires the Department of Health and Human Services to create a process for faster decisions on items and services that commonly get approved...While the bill only applies to MA plans, lawmakers hope to eventually tackle more types of insurance such as commercial plans...READ MORE