- Walgreens Files $140 Million Suit Against Theranos (thestreet.com)
Amid the election fervor...Walgreens Boots Alliance quietly took action against its former partner, Theranos, for breach of contract...The retail pharmacy filed a $140 million lawsuit against the embattled blood test device maker, suing for breach of contract…”We are disappointed that Walgreens filed this lawsuit," Elizabeth Holmes...founder and CEO of Theranos said in a statement. "Over the years, Walgreens consistently failed to meet its commitments to Theranos."...On June 12 Walgreens announced that it would terminate its relationship with Theranos and close all 40 of its Theranos Wellness Centers, which operated in Arizona. This followed the company's January announcement that it was halting Theranos lab testing operations at its Palo Alto, Calif. location.
- Pfizer’s Lyrica patent appeal fails in U.K., endangering bid to protect $5B med (fiercepharma.com)
Pfizer’s divide-and-conquer approach to Lyrica in the U.K. just hit a wall. The Court of Appeal upheld a ruling that struck down key patent claims on Lyrica and cleared Actavis’ generic of infringing it...The...case centered on a “carve-out” approval for Actavis’ Lyrica generic, a type of regulatory nod that branded drugmakers see as a threat...drugmakers want to prolong their monopoly access to patients, and they use follow-up patents to extend their protection past the time when IP coverage expires on the original compounds...some recent “skinny” regulatory nods--from the FDA as well as international regulatory agencies--that clear generics only for particular indications have complicated those efforts, because they give generics makers an entreé onto the market while so-called method-of-use patents remain in effect...The patent at issue in this case covered Lyrica’s use as a pain treatment; the patent on pregabalin itself, the active ingredient...had already expired. These days, Lyrica is used more often for pain than for its original indication as a seizure drug...The company hopes to now take its fight to the U.K. Supreme Court...Pfizer maintains its strong belief in the validity and importance...of the patent…
- European regulators appeal rulings that prevent release of drug data (statnews.com)
Once again, drug makers and European regulators are clashing over disclosing information...the European Medicines Agency is appealing two different rulings by the General Court of the European Union that prevent it from releasing data to third parties…
- ... first order blocked release of a case study report for Translarna...which is sold by PTC Therapeutics...Until PTC objected, the EMA planned to provide access to the report in response to a request, albeit with redactions that agency officials maintain are in accordance with their own regulations…
- ...second order blocked the release of three toxicity studies for Bravecto...sold by Merck’s animal health unit, which also objected to disclosure.
Our approach to transparency has been welcomed by many of our stakeholders, and these court cases are a good opportunity to test our rules on making available to the general public the documents on which EMA’s scientific opinions on medicines are based...The episode was closely watched because it arose as the pharmaceutical industry faced growing disclosure pressure following scandals over safety or effectiveness data that were not publicly shared. Drug makers have argued that disclosing certain data may compromise trade secrets or patient privacy. Consumer groups counter such information is kept out of reach at the expense of patients...
- Drug maker loses appeal of antitrust pay-to-delay case in Europe (statnews.com)
A European Commission court upheld an antitrust fine that was imposed three years ago against Lundbeck and four other drug makers for allegedly conspiring to delay the availability of a lower-cost generic version of an antidepressant...The ruling...came in response to an appeal of a 2013 decision that found Lundbeck and the generic drug makers pursued a pay-to-delay deal that violated European Union anticompetition regulations. The European Commission had fined the companies a total of $165 million with Lundbeck ordered to pay the bulk of the fine, or about $105 million...Regulators argue these deals are anticompetitive, force consumers to overpay for medicines, and escalate costs to the overall health care system. In the United States, the Federal Trade Commission estimates such deals cost Americans about $3.5 billion annually. Drug makers counter that the deals are not only legal, but allow lower-cost generic drugs to reach consumers faster than if patent litigation continued...
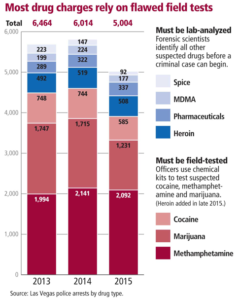
- Defense attorneys want to challenge Las Vegas police use of faulty drug tests (reviewjournal.com)SPECIAL INVESTIGATION: Las Vegas drug convictions rely on faulty police field tests (reviewjournal.com)
A prominent organization of defense lawyers in Las Vegas this week formed a committee to explore ways of challenging local law enforcement’s methods for gaining drug convictions...The committee, set up by the Nevada Attorneys for Criminal Justice, will look at the use of what are known as chemical field tests, inexpensive kits used by police and prosecutors to make drug arrests and gain guilty pleas. Officers typically drop suspicious materials into a chemical pouch and look for telltale shifts in color ostensibly meant to indicate the possible presence of illegal drugs. The tests are often the only evidence used to win convictions...The Las Vegas Metropolitan Police Department crime lab had submitted a formal report detailing the shortcomings of the tests to federal authorities in 2014, and yet to this day the lab still endorses the use of the tests in criminal prosecutions...
- SEC charges former Puma biotech exec with $1.1 million in insider trading (statnews.com)
In the latest instance of alleged insider trading in the pharmaceutical industry, a former Puma Biotechnology executive was charged with illegally making more than $1.1 million by taking advantage of confidential information about clinical trials for a cancer drug...Robert Gadimian, who was senior director of regulatory affairs, bought and sold Puma stock after learning about favorable study results for a medicine that was being tested to treat breast cancer…This alleged episode of insider trading is only the latest instance involving the pharmaceutical industry or those working with drug makers. The issue has increasingly raised concerns in connection with clinical trial work, as well as deal-making and the drug approval process, which some fear can be distorted by such activities.
- Ex-Insys sales manager arrested in U.S. fentanyl-kickback case (reuters.com)
A former Insys Therapeutics Inc district sales manager was arrested...on charges he participated in a scheme to pay kickbacks to doctors to prescribe a drug containing the opioid fentanyl...Jeffrey Pearlman...was charged in a criminal complaint filed in federal court in New Haven, Connecticut, becoming the latest individual to face prosecution in connection with probes involving Insys' drug Subsys…The charges come as Insys faces a number of state and federal investigations involving Subsys as U.S. authorities seek to combat a national epidemic of opioid abuse...Prosecutors said Pearlman and sales representatives he managed induced doctors, advanced practice registered nurses and physicians' assistants to prescribe Insys' fentanyl spray by paying them to participate in sham "speaker programs."...As a result of the scheme, federal healthcare programs incurred millions of dollars in losses, prosecutors said...
- Drug maker faces a shareholder suit for failing to tell the whole truth (statnews.com)
Memo to biopharma executives: when talking to shareholders, take care to tell the whole truth and nothing but the truth...That’s the message delivered this week to Arena Pharmaceuticals after a federal appeals court ruled...that shareholders can proceed with a lawsuit alleging the company deliberately withheld crucial information about a potential problem with a widely anticipated diet drug...At issue was a series of statements Arena executives made in 2009 about the progress they were making in winning Food and Drug Administration approval for the Belviq (lorcaserin) diet pill... company executives made or issued statements expressing confidence that the pill would be approved, because safety and effectiveness had been demonstrated through clinical trials and animal studies. Here’s the rub: while the reference to animal studies mentioned the need to ensure there was no risk that cancer seen in rats could develop in humans, Arena did not disclose a dispute about study results with the FDA...Whether the shareholders prevail remains to be seen...The ruling only means that the shareholders get to fight another day. But the court is sending a signal that if drug makers are going to describe trial results and discussions with the FDA, they should not dress up information, a common predilection in a world where exciting investors is a high priority.
- Buyers remorse or fraud? Teva and Mexican brothers slug it out over sour deal (statnews.com)
Drug makers are constantly foraging for deals to bulk up their pipelines, but not all go according to plan...Consider the nasty spat between Teva Pharmaceuticals and two Mexican brothers, Fernando and Leopoldo Espinosa...they have filed dueling lawsuits after their $2.3 billion deal went sour…At issue was a move made last year by Teva...to expand in Mexico by purchasing Rimsa, one of the country’s largest independent pharmaceutical manufacturers, from the Espinosa brothers. At the time, Teva hailed its acquisition...as a "significant platform for growth" in the second-largest market in Latin America...Teva claims the brothers engaged in chicanery, ploys, and misrepresentations about their business in order to walk away with huge profits, according to its lawsuit...Rimsa was "was engaged in a years-long scheme to sell defective and unlawful products and to conceal those violations from Mexican regulators...Teva lawsuit goes on to claim that Rimsa submitted fraudulent information to regulators about ingredients suppliers and lied about laboratory tests, including the stability tests that must ensure the stated shelf life of each product was accurate and that the product would remain stable, and, therefore, safe and effective…
- Pharma suffers a setback in battle over Ohio drug pricing ballot measure (statnews.com)
An Ohio court has given a significant boost to a controversial ballot measure that is designed to lower the cost of medicines...In a ruling...the state Supreme Court decided that thousands of contested signatures on petitions submitted to the General Assembly were valid...The 4-to-3 decision capped months of procedural and legal skirmishes over the Ohio Drug Price Relief Act, which would require the state to pay no more for medicines than the US Department of Veterans Affairs...the ballot measure was opposed by the Pharmaceutical Research and Manufacturers of America...the Ohio Manufacturers Association, and the Ohio Chamber of Commerce. The groups contested the validity of signatures on a petition that had to be submitted to the general assembly as part of the state’s two-step process to place a measure on the ballot...Some statewide organizations and health care experts are concerned that the proposal, if enacted, is unworkable and will force a lengthy and complex litigation and bureaucratic quagmire...