- FDA’s drug manufacturing oversight lags as pandemic wears on (fiercepharma.com)The FDA’s Weak Drug Manufacturing Oversight a Potentially Deadly Problem (ien.com)
Two and a half years in, the COVID-19 pandemic continues to drag on the FDA’s oversight of drug manufacturing plants...In fiscal year 2021, the FDA sent out 70% of follow-up letters to manufacturing facilities within 90 days of an inspection. Over that same stretch, the regulator completed 48% of regulatory actions for facilities in need of follow-up within six months of inspection closing, the agency said in a new report...To put those stats in perspective, the agency in fiscal year 2020 sent 78% of classification letters within the 90-day time frame and knocked out 63% of regulatory actions required over a six-month span. And in 2019, it sent out 87% of letters and completed 74% of regulatory actions during those time frames...READ MORE
- U.S. Supreme Court rebuffs opioid maker Insys founder’s conviction appeal (reuters.com)
The U.S. Supreme Court...rejected bids by Insys Therapeutics Inc founder John Kapoor and another former executive of the drugmaker to overturn their convictions for conspiring to bribe doctors to prescribe addictive opioids and defraud insurers into paying for them...Kapoor, 78, is serving a prison sentence of 5-1/2 years and is the highest-level corporate executive convicted at trial of crimes related to the opioid epidemic that has killed hundreds of thousands of Americans in the past two decades...READ MORE
- ASHP Calls for Removal of Prescribing Barriers for COVID Antivirals (ashp.org)Québec Authorizes Pharmacists to Prescribe Paxlovid (ashp.org)
The White House announced the launch of the first federally supported test-to-treat site, a part of the White House test-to-treat initiative designed to improve access to COVID-19 antiviral oral medications for certain high-risk patients. In response, ASHP sent a letter to Ashish Jha, the White House COVID-19 Response Coordinator, requesting the administration dramatically expand access to COVID-19 antivirals in all communities by removing the federal barrier preventing pharmacists from initiating therapy with these time-sensitive medications...READ MORE
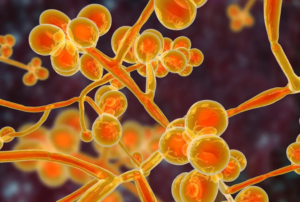
- ‘Superbug’ outbreaks reported at Nevada hospitals, nursing facilities (reviewjournal.com)Nevada hospitals with ‘superbug’ outbreaks identified (yournews.com)
State and federal health authorities are investigating ongoing outbreaks at Nevada hospitals and nursing homes of a drug-resistant “superbug” that can lead to serious illness and even death...As of mid-April, the Nevada Department of Health and Human Services has been investigating outbreaks of a fungus called Candida auris at acute-care hospitals, long-term acute-care hospitals and skilled nursing facilities, according to a technical bulletin sent by the state to health care providers...READ MORE
- Pfizer stock-owning judges play musical chairs in New York City vaccine mandate lawsuits (fiercepharma.com)Judge Caproni Recuses Herself from NYC Vaccine Mandate Lawsuit because she owns Pfizer stock (teachersforchoice.org)
What do you do when you can’t find a judge who doesn’t own Pfizer stock? Keep looking. Then look again—and again...Such was plaintiffs’ plight this week in two consolidated federal lawsuits taking aim at the New York City Department of Education’s COVID-19 vaccine mandates. Since Monday, two judges have come and gone, thanks to their financial ties to pandemic vaccine companies. Meanwhile, a third has vowed to stick around, arguing her investments in Pfizer and Johnson & Johnson are out of date...READ MORE
- US judge keeps Nevada execution challenge alive, for now (apnews.com)
A federal judge declined Monday to either decide or dismiss a condemned Nevada killer’s lawsuit challenging the constitutionality of the state plan for what would its first lethal injection in more than 16 years...U.S. District Judge Richard Boulware II left open Zane Michael Floyd’s case — at least for now — and set an Oct. 11 date for attorneys representing Floyd and the state to return to court in Las Vegas...The judge said he might still close the matter in coming weeks for what he termed “mootness,” since state prison officials testified that they do not have the drugs they would need to conduct an execution. The prison supply of the sedative ketamine expired Feb. 28, and officials said they’ve had trouble procuring more...READ MORE
- COVID-19 Federal probe of COVID testing company with stunning error rate expands to Nevada (thisisreno.com)
Federal authorities are expanding an investigation into Chicago-based Northshore Clinical Labs following a ProPublica story that raised questions about its COVID-19 testing operations in Nevada...an investigator with the Inspector General’s Office of the U.S. Department of Health and Human Services indicated he planned to subpoena documents from Nevada health officials...“Myself and other law enforcement agencies have had a case opened regarding Northshore Clinical Lab for quite some time,” wrote Special Agent Peter Theiler, who is based in Chicago. “After reading the ProPublica article on Northshore Clinical Lab regarding Nevada patients, we are interested in obtaining records related to testing for COVID-19 for Northshore Clinical Lab rapid test results and PCR test results for Nevada.”...READ MORE
- Greece files €214M bribery suit against Novartis (fiercepharma.com)
The anticipated Greek lawsuit against Novartis has been filed, with the country asking for 214 million euros in compensation from the pharmaceutical giant...Greek Health Minister Thanos Plevris said in a statement that the Greek state is seeking compensation for the damage it has suffered “from the actions that Novartis itself has admitted to in the USA that concerns payments to doctors.” The minister added that the state reserves the right to claim any damage it has suffered with a newer lawsuit and is clear that “all sanctions against Novartis for its illegal practices will be applied,”...READ MORE
- After Aduhelm controversy, Congress looks to strengthen FDA’s accelerated approval powers: WSJ (fiercepharma.com)
...Biogen went from winning accelerated approval for its Alzheimer’s drug, Aduhelm, to all but abandoning the controversial medicine. Now, Congress is weighing changes to the FDA program that triggered the entire episode...The proposals are designed to give the FDA more control over the accelerated approval program...The approval pathway is designed to speed up therapies for patients with few or no other options, but drugs approved under the program have to go through a postmarketing trial to confirm its benefits...Accelerated approval reform has taken center stage in the wake of the Aduhelm debacle. Last June, the agency approved the drug based on the surrogate endpoint of reducing amyloid beta plaque in the brain. At the time, the agency said a positive effect on the endpoint was “reasonably likely to predict a clinical benefit to patients.”...A controversy ensued, and in a high-profile decision earlier this year, the Centers for Medicare & Medicaid Services restricted coverage to patients in approved clinical trials. The decision amounted to a severe throttling of the rollout...READ MORE
- Police charge big pharma boss with falsifying his Covid vaccination status (sott.net)
Jose Maria Fernandez Sousa-Faro, president of European pharmaceuticals giant PharmaMar, has been charged by police with being falsely vaccinated against Covid-19. Dr. Sousa-Faro has been caught up in a scandal in Europe involving people being added to the National Immunization Registry in exchange for large sums of money...Police allege that Sousa-Faro arranged to be injected with a saline solution instead of a Covid-19 vaccination and paid thousands of dollars to have his name added to Spain's immunization register, as confirmed by police sources and reported by El Periodico de Espana...READ MORE