- Merck, insurers advance fight over cyberattack-related coverage to New Jersey Supreme Court (fiercepharma.com)
That didn't take long. About a month and a half after Merck & Co. scored a legal win in the insurance case tied to the 2017 "NotPetya" cyberattack, the case is in appeals—again...The case, Merck & Co., Inc. v. Ace American Insurance Company, is heading to the New Jersey Supreme Court...This development follows a lower court's ruling in May rejecting the insurers' argument that the “hostile/warlike action” exclusion clause should apply in this case. In making that ruling, the New Jersey appellate court said the exclusion shouldn’t be applied to a cyberattack on a non-military company—even if it originated from a government or sovereign power...After the 2017 cyberattack, tens of thousands of computers in Merck's global network were infected, leading to a significant disruption in the pharma company's business...READ MORE
- National cancer group reports widespread chemo shortages, calls on government and industry to help resolve them (fiercepharma.com)
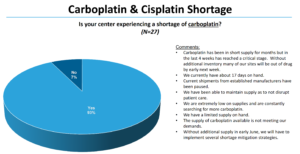
As pharma supply chain problems drag on, a shortage of key cancer drugs has afflicted a large number of treatment centers and many patients. Now, a leading treatment center group is putting more statistics behind the shortage...Late last month, the National Comprehensive Cancer Network Best Practices Committee conducted a survey (PDF) of 27 member centers across the U.S. The group found that nearly all treatment centers, or 93%, reported a carboplatin shortage. In addition, 70% of the centers reported a cisplatin shortage...READ MORE
- California lawmakers greenlight $150M of emergency loans for struggling hospitals (fiercehealthcare.com)
California lawmakers have fast-tracked legislation to loan out $150 million to hospitals across the state struggling to stay open with promises to provide further support when finalizing the state’s budget next month...The bill, passed Thursday in California’s senate and assembly, AB-112, would establish a Distressed Hospital Loan Program through Jan. 1, 2032. The program would provide interest-free loans to nonprofit and public hospitals “in significant financial distress,” as well as to “governmental entities representing a closed hospital to prevent the closure or facilitate the reopening of a closed hospital.”...If passed by Gov. Gavin Newsom, who has signaled his support, the bill instructs the state Department of Health Care Services and the state Department of Public Health to develop a methodology for handing out the loans “that shall consider factors” such as whether the hospital is small, rural, a critical access hospital or treating a disproportionate share of Medicaid patients, among other factors. Facilities will be excluded from consideration if they’re part of a system with more than two hospitals, investor-owned hospitals and free-standing inpatient psychiatric hospitals...READ MORE
- One killed, 4 injured in Atlanta medical office mass shooting (fiercehealthcare.com)
Four people were injured and one woman was killed in a mass shooting Wednesday in a downtown Atlanta medical office waiting room, local law enforcement said...The suspected gunman, a 24-year-old man, entered Midtown medical building and shot the victims around noon, police said. He escaped in a stolen vehicle, kicking off a manhunt before he was taken into custody without incident later that evening, they said...The victim who was killed has been identified as Amy St. Pierre, a 39-year-old who worked for the Centers for Disease Control and Prevention, according to a statement from the agency...READ MORE
- Thousands of Nevadans’ health coverage in limbo as Friday Health Plans placed in receivership (nevadacurrent.com)
Nevada Insurance Commissioner Scott Kipper filed for regulatory supervision of Friday Health Plans of Nevada, which has over 2,800 individuals in the state, after the company announced last week that it will “wind down…business operations.”...“Unfortunately, Friday has been unable to scale our financial infrastructure to match the pace of our growth and secure the additional capital required to run our business,” the company said in a statement on its website last week. “While we are deeply disappointed, we agree with the decision of our State regulators that it is necessary to wind down Friday’s business operations over time in accordance with the regulations in the states where we are operating.”...READ MORE
- U.S. drug shortages highlight dependence on China, gray supply chains (thechinaproject.com)
The U.S. Food and Drug Administration is loosening restrictions to allow the Chinese company Qilu Pharmaceuticals to import cisplatin, a cancer medicine currently in short supply...The emergency move to import Qilu’s cisplatin, which is not FDA-approved, comes as U.S. hospitals ration chemotherapy drugs that can dramatically improve a patient’s prognosis. An FDA official told The China Project that the agency is exploring continued importation of cisplatin and temporary importation of another cancer drug, carboplatin, but, when asked, wouldn’t provide details on plans for further temporary importation from China. This is the first time the U.S. has allowed for temporary importation of cisplatin, the FDA official said...READ MORE
- FDA, weighing Perrigo’s OTC birth control application, raises questions about real-world use (fiercepharma.com)
As a long-awaited advisory committee meeting on Perrigo’s over-the-counter birth control prospect Opill nears, the FDA says many big questions remain. Chief among them: Will people use the drug as intended in the real world?...Ahead of this week’s joint expert panel meeting, the FDA released briefing documents posing two main questions for its expert committees: First, just how likely are consumers to use Opill “in an effective and safe manner,” relying solely on the nonprescription prospect’s label and without help from a healthcare professional?...Second, will consumers who shouldn’t use the product avoid the temptation?...READ MORE
- After nationwide deal, Teva reaches $193M opioid settlement with holdout Nevada (fiercepharma.com)Nevada reaches $193M settlement in latest opioid lawsuit (reviewjournal.com)
When Teva proposed its sweeping $4.25 billion opioid settlement to resolve thousands of claims across the country, all U.S. states except for Nevada and New Mexico jumped on board. Now, the company has worked out a separate $193 million deal with one of the holdouts...Under the deal, the generics giant will make annual payments to Nevada on a sliding scale starting next July and lasting through July 2043. The payouts will start at $7 million and rise to $9 million through 2037, then increase to $27 million in 2042...The cash will be divvied between Nevada and members of the One Nevada Agreement on Allocation of Opioid Recoveries, a group formed to distribute opioid-related funds to local governments...READ MORE
- Ahead of high-stakes California trial, GSK notches Zantac win in Canada (fiercepharma.com)GSK was warned repeatedly about Zantac impurity but played down risks: Bloomberg (fiercepharma.com)
As GSK's July court date nears for a key Zantac trial in California, the company can wipe its hands of at least one Canadian class action suit...The company said in a Friday statement that it “welcomes the decision” of the British Columbia Supreme Court to dismiss a proposed class action suit on behalf of Canadian Zantac users...A Vancouver man filed the lawsuit in 2020, alleging that his use of the heartburn med from 2018 to 2019 caused him to develop cancer. His complaint named more than a dozen companies as defendants, including Sandoz Canada and GSK...But the court dismissed the case due to “the uncontroverted evidence that neither ranitidine nor NDMA are reliably associated with increased cancer risk,” GSK said in its statement...READ MORE
- Telehealth providers cheer DEA move to temporarily extend virtual prescribing flexibilities (fiercehealthcare.com)
The clock is ticking on the end of the COVID-19 public health emergency and, with it, telehealth flexibilities that enabled doctors to virtually prescribe controlled medications to their patients...Facing major backlash to its proposed rules released in February, the Drug Enforcement Administration is looking to buy some time to reconsider whether it should enforce stricter limits around the prescribing of controlled substances via telehealth...The Biden administration said at the time that the new rule seeks to provide safeguards to prevent online over-prescribing of controlled medications. Teleprescribing has been touted as a robust tool for bringing medications for opioid use disorder to rural areas in the ongoing treatment of the opioid epidemic...READ MORE