- FDA warning letter smacks Allay Pharma for potency problems, API testing issues (fiercepharma.com)
Florida-based drugmaker Allay Pharmaceuticals is in hot water with the FDA after it failed to right the manufacturing wrongs flagged in an inspection last May...FDA slapped Allay for failing to set up proper procedures and process controls for tablet manufacturing at its plant in Hialeah, Florida, according to a warning letter...Inspectors turned up potency problems, substandard API testing and biologics manufacturing without FDA approval...READ MORE - Nearly 60% of Critical Care Pharmacists Report Burnout (pharmacypracticenews.com)
With many ICUs reaching or exceeding maximum capacity in spring 2020 due to the COVID-19 pandemic, it may not be a surprise that about 60% of critical care pharmacists reported feeling burned out in a recent national survey...In an electronic survey that queried critical care pharmacists about their institutions and number of activities performed in May and June 2020, 128 of 221 respondents (58%) reported feelings of burnout such as emotional exhaustion and depersonalization...READ MORE
- FDA clears Lilly’s COVID-19 antibody cocktail for emergency use (biopharmadive.com)
The Food and Drug Administration...cleared an antibody drug cocktail from Eli Lilly for emergency use for treating people recently diagnosed with COVID-19...The cocktail, a combination of two coronavirus-targeting antibodies, is authorized only for people with mild or moderate symptoms of COVID-19, but who are at high risk of the disease's worst effects due to age, underlying medical conditions or other preexisting conditions...The combination pairs Lilly's bamlanivimab...with another antibody called etesevimab that the drugmaker developed in partnership with China's Junshi Biosciences. Each antibody targets a separate section of the "spike" protein used by the SARS-CoV-2 virus to breach the body's cells — a feature designed to preserve the drug's effectiveness even as the virus mutates...READ MORE
- No need ‘to start at square one’: FDA plans to lay out a speedy path for COVID-19 vaccines, drugs against variants (fiercepharma.com)
New coronavirus variants have prompted COVID-19 vaccine makers to start developing updates to their existing offerings. To speed their journey to a pandemic-fatigued public, the FDA says it’s developing expedited review rules for the follow-up shots...The FDA’s working on guidance for the types of data needed to support changes to COVID-19 vaccines. The new rules would provide for “streamlined clinical programs” that can demonstrate an immune response to new variants and “can be executed quickly,” FDA’s acting commissioner, Janet Woodcock, said in a statement...READ MORE
- PwC joins Medicines Manufacturing Innovation Centre (outsourcing-pharma.com)
CPI, one of the founding partners in the Medicines Manufacturing Innovation Centre, has announced the signing of an agreement with PwC, making the company the latest partner in the collaborative effort. The Centre (established via a partnership among CPI, the University of Strathclyde, UK Research and Innovation, Scottish Enterprise and founding industry partners AstraZeneca and GSK) seeks to promote more innovative drug manufacturing processes and enable a more agile supply chain...PwC and the existing partners will work to maximize technological opportunities across the pharma supply chain. Efforts include...projects, intended to advance emergent and disruptive technologies. The program is partially funded by Innovate UK through the Industrial Strategy Challenge Fund and Scottish Enterprise via the Scottish Government...READ MORE
- Nevada climbs out of bottom in administering vaccine, CDC says (reviewjournal.com)
Nevada no longer has one of the worst COVID-19 vaccination rates per capita in the U.S., according to federal data...The Silver State had consistently ranked among the bottom five states at administering vaccine for weeks. It now ranks 12th worst, the Centers for Disease Control and Prevention reported...READ MORE
- New Massachusetts law recognizes pharmacist as providers (pharmacist.com)
...the governor of Massachusetts signed legislation that includes language recognizing pharmacists as health care providers in the state...The new law, S.2984: An Act Promoting a Resilient Health Care System That Puts Patients First, not only expands scope of practice for certain health care providers, but also increases insurance coverage for telehealth services...“This is a culmination of nearly a decade of advocating for pharmacists and their profession in our state,” said Massachusetts Pharmacists Association President Maureen Judkins, PharmD...READ MORE
- Fresenius Kabi unit admits it hid records from FDA inspectors—and settles with DOJ for $50M (fiercepharma.com)
Fresenius Kabi Oncology fell afoul of the FDA in 2013 when the agency discovered employees had hidden records before a manufacturing inspection. Now, the drug ingredients manufacturer is admitting fault—and has reached a deal with the U.S. Department of Justice to put the investigation to bed...The Fresenius Kabi unit agreed to pay a $30 million fine, forfeit another $20 million and plead guilty to concealing and destroying records ahead of a 2013 FDA inspection in Kalyani, India...READ MORE
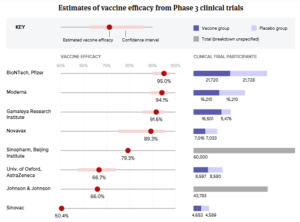
- The first coronavirus vaccines have arrived. Here’s where the rest stand. (biopharmadive.com)
Study results showed vaccines from J&J; and Novavax to be effective against COVID-19. But seemingly weaker protection versus new virus variants have raised concerns...Scientists, drugmakers and governments have moved with unprecedented haste to develop a vaccine against the new coronavirus...The fastest of them have completed studies proving their vaccines can protect against COVID-19. A half dozen shots from developers in the U.S., U.K., Germany, China and Russia have now been cleared by regulators for emergency use...READ MORE
- Pfizer, Johnson & Johnson balk at shareholders’ push for COVID-19 vaccine pricing info (fiercepharma.com)
What's the rationale behind COVID-19 vaccine and drug prices? You don’t have a need to know—or so say a couple of the leading contenders...Two major players in the pandemic fight, Pfizer and Johnson & Johnson, are urging the Securities and Exchange Commission to forestall shareholder resolutions that would require them to disclose how they set prices on their COVID-19 vaccines...Several not-for-profit groups are pushing the two companies—along with fellow pharmas Eli Lilly, Gilead, Merck & Co. and Regeneron—for information on their drug and vaccine pricing decisions, citing the federal money all have received, either for supplies, R&D or manufacturing scale-up. Or all three...READ MORE