- States Move to Ban Accumulators (drugtopics.com)The State of Employers’ Pharmacy Benefits: A Review of 2022 and the 2023 Outlook for Copay Programs (drugchannels.net)How Copay Accumulators and Maximizers Have Changed Payers’ View of Copay Support (drugchannels.net)
Sixteen states have banned a pharmacy benefit management practice that involves not counting the value of drug copay assistance from manufacturers toward patient deductibles...Drugmakers use copay assistance programs to shield patients from out-of-pocket expenses — and build market share for their products in the process. But pharmacy benefit managers have cried foul, saying the copay programs undercut formularies and wind up increasing the use of expensive drugs that are not any better than less expensive ones. They have pushed back with “copay adjustment programs,” especially “copay accumulators,” which are designed to blunt the effect of the copay assistance programs by not counting their value toward patient deductibles...READ MORE
- Fierce Pharma Politics—PhRMA sues Trump administration over importation order (fiercepharma.com)Statement on Litigation Challenging Legality of the Administration’s Most Favored Nation Rule (phrma.org)
It's no secret that the drug industry isn't thrilled with the Trump administration's last-ditch push to bring down drug prices, including an order allowing drug imports from Canada. Now, PhRMA is taking its objections to court...After the administration unveiled its importation executive order in final form in September, PhRMA and other groups sued the Department of Health and Human Services last month arguing the measure violates the Food, Drug, and Cosmetic Act...The lawsuit claims HHS’ importation effort increases risks for the U.S. drug supply chain and forces companies to disclose trade secrets...READ MORE
- Aetna, city of New Haven hit with OCR fines after data breach (healthcareitnews.com)
The U.S. Department of Health and Human Services' Office for Civil Rights leveraged $1,000,000 in fines against Aetna Life Insurance Company and $202,400 against the city of New Haven, Connecticut, to settle potential HIPAA violations...The fines are just the latest moves on the part of OCR to enforce HIPAA regulations around protected health information..."When individuals contract for health insurance, they expect plans to keep their medical information safe from public exposure. Unfortunately, Aetna's failure to follow the HIPAA Rules resulted in three breaches in a six-month period, leading to this million dollar settlement," said OCR Director Roger Severino in a statement...READ MORE
- Nevada reverses ban on rapid tests after federal pushback (apnews.com)Nevada's chief medical officer not licensed to practice medicine in U.S. (washingtontimes.com)
Nevada health officials said they would resume the use of rapid “point of care” tests after federal health officials chided them for banning their use and accused them of violating federal law...Dr. Ihsan Azzam, Nevada’s chief medical officer, doubled down on his insistence that too many questions remained about the accuracy of rapid antigen tests. He said his team was “disappointed” in the U.S. Department of Health and Human Services...“We are not saying the tests have no use, we are just saying pause for further review and additional training,”... Admiral Brett Giroir, assistant secretary for health at the U.S. Department of Health and Human Services, told reporters that federal law prohibits states from imposing a ban like the one that Nevada health officials ordered Oct. 2. He said Nevada is the only state to do so...READ MORE
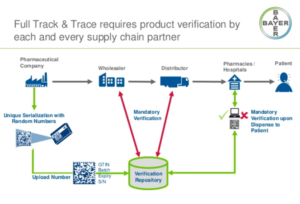
- Few Rx supply chain stakeholders prepared to share DSCSA-required transaction data (chaindrugreview.com)
Results from a new HDA Research Foundation survey indicate that the pharmaceutical supply chain is entering a critical phase in achieving the transaction data connections required to comply with the Drug Supply Chain Security Act in 2023...The DSCSA requires transaction data with product identifiers to be provided with physical product on November 27, 2023...“many healthcare supply chain trading partners are realizing there is work to be done to establish proper business-to-business connections; ensure data are formatted, transmitted and received successfully; that processes for troubleshooting are created; and that products in inventory have the right data attached to them for shipping after November 27,” said Perry Fri...COO of the HDA Research Foundation. “The Foundation’s survey shows...what might be leading to slow implementation rates across the supply chain.”...READ MORE
- Track-and-trace requirements go into effect on Friday (pharmacist.com)
Starting Friday, November 27, 2020, pharmacies must buy and sell only products with a required “product identifier” on their packages. This requirement is part of the phased-in implementation of the Drug Supply Chain Security Act of 2013, also known as the “track-and-trace” law. By Friday, dispensers should be familiar with this requirement and know what to do if a product identifier is not on the package when they receive products that require it. The product identifier is on most drug packages in both human-readable format and on a machine-readable 2D data matrix barcode...“The challenge for dispensers is that not all drug product packages are required to have a product identifier, and there is no central database to check if a product should have one,”...READ MORE
- Eli Lilly faces FDA crackdown for manufacturing issues at plant handling COVID-19 antibody (fiercepharma.com)
Just days after Eli Lilly sent an COVID-19 antibody ahead for FDA scrutiny, the drugmaker's immediate chances were halted by a damaging trial stop for the therapy. Now, the FDA has knocked Lilly for its manufacturing controls at a New Jersey site producing the antibody...The FDA cited Lilly's Branchburg, New Jersey, facility on two counts of inadequate "control of computer systems," Lilly confirmed in an email. Those two findings included deleted data on the company's manufacturing processes and failed quality control over audit paper trails...FDA investigators flagged the quality control deficiencies in a notice...The investigators' findings qualified as official action indicated (OAI) in the notice, the highest enforcement priority level from the FDA for its observations...READ MORE
- Most Americans Oppose Biden’s Prescription Drug Price Controls (realclearhealth.com)
In response to U.S. prescription drug spending rapidly outpacing inflation, reaching an estimated $358.7 billion in 2020, President Biden last week demanded that Congress adopt strict federal price controls on prescription drugs...to cap annual out-of-pocket drug spending for Medicare beneficiaries, empowering the Secretary of Health and Human Services to choose pharmaceutical winners and losers, and financially crippling penalties for pharmaceutical companies that don’t acquiesce to price controls — are likely to have disastrous effects on the availability of critical medications. What’s more, they’re deeply unpopular with the overwhelming majority of Americans, and could prove to be a significant fiscal and health liability for our nation in the years to come...READ MORE
- Pharmacies must comply with certain track-and-trace requirements by November 27, FDA says (pharmacist.com)
Despite APhA’s concerns that compliance with certain Drug Supply Chain Security Act requirements undermines pandemic response efforts, FDA has confirmed that “dispensers,” including pharmacies, must meet a requirement that goes into effect on November 27, 2020. APhA and pharmacy partners had requested that FDA delay enforcement of certain portions of the DSCSA—also known as “track and trace”—mandate...READ MORE
- Nevada Board of Pharmacy Newsletter October 2020 (bop.nv.gov)
- Pharmacists’ Vital Role in Influenza Immunization in Nevada
- Proposed Amendments to Existing Regulations Permitting Pharmaceutical Technicians to Administer Immunizations
- National Pharmacy Compliance News
- FDA Recommends Health Care Providers Discuss Naloxone With Patients Receiving Opioids, OUD Treatment
- Proposed Rule to Require Electronic Submission of DEA Form 106
- Inappropriate FentaNYL Patch Prescriptions at Discharge for Opioid-Naïve, Elderly Patients
- SAMHSA Health Privacy Rule Revised to Better Integrate, Coordinate Care for Patients With SUD