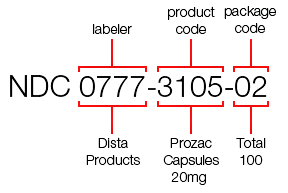
- The NDC Shortage: What the FDA Could (and Should) Do to Address It (drugchannels.net)An Open Letter To The FDA: New NDC Format Public Meeting (rxtrace.com)
...the U.S. Food & Drug Administration will run out of 5-digit National Drug Code codes within the next ten to fifteen years…The problem now facing U.S. healthcare is that, due to an explosion of new labelers entering the market, the FDA is running out of 5-digit labeler codes. The 5-digit format provides 90,000 potential combinations, and although that seems like a lot, the FDA anticipates running out of labeler codes within the next 10-15 years...the disruption and impact to the healthcare industry will be widespread and of a large magnitude, and will require retooling of major industry systems...
- FDA plan would ease regulations for prescription drug apps (biopharmadive.com)
The Food and Drug Administration is seeking public comment on a proposed framework for regulating software applications developed by drugmakers for use in conjunction with their prescription drug products...The new approach would treat most prescription drug apps, including dose calculators, symptom trackers and medication reminders, as promotional labeling...drugmakers would need only to submit to the agency copies of the content of what the apps display to consumers, following existing reporting requirements for promotional materials...In other cases, such as when a drugmaker wants to show that software has an effect on a clinical outcome and wants to include information about the software in the FDA-required drug labeling, prior FDA approval would be required...
- CMS finalizes site neutral payment rule (healthcarefinancenews.com)
The Centers for Medicare and Medicaid Services has finalized site neutral payments in the hospital outpatient prospective payment system and ambulatory surgical center payment system rule...CMS is using site neutral payments to level the playing field between what physician offices and hospital outpatient departments are paid for certain clinical visits...In addition...CMS is expanding its 340B policy by extending the payment change to additional off-campus provider-based hospital outpatient departments that are paid under the physician fee schedule...Today's rule advances competition...for providers so they can compete for patients on the basis of quality and care...The final policies remove unnecessary and inefficient payment differences so patients can have more affordable choices and options.
- CMS eyes Part D catastrophic coverage reforms that would shift risk to insurers (fiercehealthcare.com)
The Trump administration is looking for ways to change the Medicare Part D drug program that would shift more risk to plan sponsors when members reach the final catastrophic coverage stage...The catastrophic coverage tier of Part D kicks in when a member reaches $5,000 in out-of-pocket costs. At that point, patients pay a flat fee or 5% of the negotiated retail drug cost, and the federal government picks up 80% of the remaining costs and plan sponsors pay 15%...That could change. Catastrophic coverage under Part D was initially developed to encourage private insurers to participate, Centers for Medicare & Medicaid Services Administrator Seema Verma said...A lot has changed since then, and now it’s “one example where Part D could be updated or modernized.”...We’re at the point now where it would be better for Part D plans to manage that portion of the benefit,” she said. “That way we could bring competition and negation to that piece as well.”
- As state awaits data from diabetes drug manufacturers, initial report highlights price increases (thenevadaindependent.com)
Pharmaceutical companies are preparing to submit their initial reports detailing why rising prices of some diabetes drugs have outpaced medical inflation, giving state officials the first detailed look into the costs associated with a disease that affects about a tenth of Nevadans...Despite a protracted legal battle...pharmaceutical manufacturers are required to submit reports...to comply with a new diabetes drug transparency law… the state released a list of 175 so-called national drug codes...manufacturers...are required to submit reports to the state detailing the factors that contributed to the price increases...
- Draft Brexit withdrawal agreement: what does it mean for the pharma industry? (pharmaceutical-technology.com)Patients and industry fret over drug supplies, if no Brexit deal (reuters.com)
...UK Government and the European Commission published a draft Brexit withdrawal agreement. Like with every industry, the document has implications for the pharmaceutical industry, including clarification about the lack of disruption to supplies during the transition period and sharing of marketing authorisation information between the UK and EU member states...A key area of debate during the negotiation process was the impact of Brexit on the pharmaceutical industry, particularly the disruption in supply of medicines...The draft agreement stipulates that the transition period will last from 29 March 2019 until 31 December 2020. During this time the UK will continue to abide by all EU rules and remain under the jurisdiction of the European Court of Justice...The aim of the transition is to provide time for the UK to negotiate its relationship with the EU, particularly in terms of trade deals...the UK will share the “marketing authorisation dossier of a medicinal product” authorised by its Medicines and Healthcare products Regulatory Agency with EU member states or the European Medicines Authority...
- Trump administration unveils plan to lower Medicare Part B prices by basing costs on other countries’ pricing (fiercehealthcare.com)Trump administration faces uphill battle in convincing provider skeptics to back new drug pricing plan (fiercehealthcare.com)
President...Trump...announced a new payment model that would more closely align the cost of Medicare Part B drugs with the prices paid for the same drugs in other countries...As they unveiled the "International Pricing Index" model, federal health officials projected the proposal could save Medicare more than $17 billion over the course of a five-year pilot. The Centers for Medicare & Medicaid Services currently pays the average sales price plus 6% for Part B drugs, and in the new proposal would instead pay a target price based on international prices and look to alternatives for the add-on fee...Medicare currently pays about 180% more than other countries for drugs in Part B, which includes pharmaceuticals that are administered in a clinical setting, HHS Secretary Alex Azar said. Through the IPI model, HHS is looking to bring that figure down to 126%.
- Nevada Medicaid to pause enrollment of mental health providers (reviewjournal.com)How Nevada Medicaid struggles with mental health care fraud (reviewjournal.com)
Nevada Medicaid officials are putting a temporary stop on the enrollment of some mental health providers while new certification requirements are considered...the largely federally funded state agency will put a six-month pause on enrolling new qualified behavioral aides and qualified mental health associates...State mental health groups had brought up concerns that some enrolled as either provider type were performing services without proper qualification…Nevada Medicaid acting Administrator Cody Phinney also expressed concern over potential improper billing for codes including medication training and support and crisis intervention...
- HHS recommended that the DEA make kratom a Schedule I drug, like LSD or heroin (statnews.com)
The Department of Health and Human Services has recommended a ban on the chemicals in kratom that would make the popular herbal supplement as illegal as heroin or LSD…HHS asserted in a letter to the Drug Enforcement Administration that two chemicals in kratom should be classified as Schedule I substances... FDA...has said that kratom is “an opioid” and has been “associated” with dozens of deaths...Kratom should not be used to treat medical conditions, nor should it be used as an alternative to prescription opioids...Some states have already banned kratom, but it’s currently legal at the federal level. It’s sold in different forms, including dry powder and capsules. According to the American Kratom Association, millions of Americans use the substance.
- Jeff Sessions announces more prosecutors for crackdown on opioid providers (fiercehealthcare.com)
A day after President Trump signed a massive piece of legislation responding to the opioid crisis into law, the Department of Justice announced it's pouring even more resources into its crackdown on prescribers...Attorney General Jeff Sessions said his agency was creating a new Appalachian Regional Prescription Opioid Strike Force to focus on communities "hit especially hard by addiction and opioid fraud."...a dozen prosecutors and data analysts will operate out of hubs in Cincinnati/Northern Kentucky and Nashville, Tennessee, monitoring the Appalachian region for fraudulent opioid prescriptions by physicians...