- FDA’s continuing use of ‘black box’ for antidepressants ignores the harms of this warning (statnews.com)
The Food and Drug Administration’s “black box” warnings and advisories give important safety information about drugs. But they can sometimes go too far and harm more people than they help...Take the FDA’s highly publicized warnings that taking antidepressants increases the risk of suicidality...among children, adolescents, and young adults. We have evidence, as do many others, that these warnings have decreased youths’ access to mental health care and increased suicide attempts...In October 2004, the FDA required a so-called “black box warning” of this risk to be printed on the labels of all antidepressant drugs. It was implemented in January 2005. Two years later, the FDA extended the same warning to include young adults, again based on industry studies...There’s no question that antidepressants can cause harm if used inappropriately. But the FDA failed to recognize — and still won’t acknowledge — that the harms of its public advisories and black box warning on antidepressants for young people more than outweigh the benefits...
- FDA confronts its limits in push on drug pricing (biopharmadive.com)
Legally, the agency has few levers to pull when it comes to addressing prices. But that hasn't stopped Commissioner Scott Gottlieb from taking a more spirited and vocal approach than past FDA chiefs. The effort comes as eye-popping price tags, some spurred by groundbreaking new treatments but others by manipulation of the drug pricing system, have prompted public and political opposition to rising drug costs...Gottlieb has aggressively used public statements to call out drugmakers for blocking or impeding market competition. And, under his leadership, the FDA has continued to ramp up approval of generic and biosimilar drugs, while pushing the boundaries of regulatory flexibility.
- Indirect effect..."Where the FDA does have authority to get involved is in how much, in what way, and how aggressively it approves drugs, or their generic or biosimilar competitors."...While it's an indirect effect, approving low-cost competitors more quickly can be a powerful force for lowering prices.
- Biosimilars showcase hurdles facing FDA...speeding competitors to market can only go so far if other forces block lower-cost rivals from reaching patients. To date, that's exactly what's played out with biosimilars...Gottlieb has expressed frustration that the FDA's steady increase in approving biosimilars hasn't resulted in greater savings. "Competition is, for the most part, anemic,"
- Naming and shaming...Gottlieb has made full use of his public platform to call out market "shenanigans" by drugmakers...Gottlieb received a lot of media coverage by attacking such practices as "regulatory gaming," and has since kept up pressure on drugmakers through public statements.
- Worth cooperating?...As Health and Human Services Secretary Alex Azar and Gottlieb continue to criticize pricing and anticompetitive practices, drugmakers are faced with a choice: ignore the growing chorus or cooperate.
- Nevada Medicaid approves policy requiring prior approval after 5 therapy sessions (thenevadaindependent.com)
Nevada Medicaid approved a requirement that therapists receive prior approval before providing more than five therapy sessions to a patient...The new policy, which takes effect on Oct. 1, will require psychologists, therapists and other mental health providers to submit written documentation to the state’s third-party vendor demonstrating the medical necessity of treatment and receive prior approval to continue providing both talk therapy and neurotherapy services after five sessions with a patient. The final policy is a scaled back version of earlier proposals from Medicaid to require prior authorization before the first session or after three sessions, both which received significant pushback from the mental health community over the last few weeks...The policy will only apply to patients enrolled in Medicaid’s fee-for-service program, in which Nevada Medicaid reimburses individual providers for services rendered, and not those who are covered under Medicaid managed care, where the state pays an insurance company a flat fee to provide health services to a patient. About one in four of the 650,000 Nevadans on Medicaid are enrolled in the fee-for-service-program.
- Brexit Guidance: MHRA Outlines What to Expect (raps.org)
The UK’s Medicines & Healthcare products Regulatory Agency...explained what pharmaceutical and medical device companies can expect during the period that the Brexit agreement is implemented...During the implementation period, which will end in December 2020, pharmaceutical firms will be able to continue UK batch release testing and Qualified Person certification in the UK, which will be recognized by the EU and vice versa, MHRA said...But one of the setbacks is that for medicines, the UK will not have voting rights in EMA and EU committees during the implementation period, MHRA said...“Marketing authorisation holders and qualified persons for pharmacovigilance will continue to be able to be based in the UK and access EU markets. There will be continued mutual recognition of manufacturing and distribution licences, as well as associated inspections such as good manufacturing practice (GMP),” the regulator added.
- Drug supply post-Brexit may occur at the expense of pharma R&D (pharmaceutical-technology.com)
With less than a year left for the UK to leave the EU, ensuring an undisrupted flow of medicines in between the UK and the EU following Brexit is a growing concern among drug developers and regulatory agencies...On 23 August the UK’s Department of Health and Social Care has asked pharmaceutical companies that supply drugs to National Health Service patients to ensure a minimum of six weeks of additional supply of medicines over and above their usual buffer stocks, due to a potential disruption of medicine imports from the EU in the event of a no-deal Brexit. While the UK still may remain part of the EU’s drug approval system following Brexit, the uncertain regulatory environment post-Brexit is urging drugmakers to prepare for the worst...The actions that will need to be implemented by the pharmaceuticals industry, such as stockpiling of medicines, duplicating facilities in both the UK and the EU area, and establishing alternative trade routes are expected to be highly costly measures...drugmakers are on their own to implement these changes, and recent survey results reported by the EMA indicate that some drugmakers have not taken sufficient steps to maintain the continuous supply of their products following Brexit.
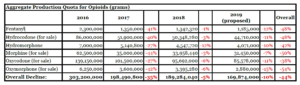
- Trump administration proposes production quota cuts for six opioids (reuters.com)Proposed Aggregate Production Quotas for Schedule I and II Controlled Substances and Assessment of Annual Needs for the List I Chemicals Ephedrine, Pseudoephedrine, and Phenylpropanolamine for 2019 (justice.gov)
The Trump administration on Thursday proposed that U.S. drugmakers cut production quotas of the six most abused opioids by 10 percent next year to fight a nationwide addiction crisis....the U.S. Justice Department and Drug Enforcement Administration said the proposed cut would be in keeping with President Donald Trump’s effort to cut opioid prescription fills by one-third within three years...
- FDA OKs first generic under new approval pathway (drugstorenews.com)
This new approval pathway was created to expedite the development and review of a generic drug for products that lack competition...The FDA gave the nod for Apotex’s potassium chloride oral solution...“Today’s approval marks the successful implementation of a new program designed to encourage generic drug development for products with inadequate generic competition,” said FDA commissioner Scott Gottlieb, in a press statement...“The quick implementation of this new pathway is part of our broader effort to foster generic competition and help address the high cost of drugs. So are our efforts to narrow the time it takes for generic drugs to reach the market by reducing the number of review cycles that generic applications typically undergo. This new generic drug application was also approved in its first cycle of review. This approval demonstrates that the competitive generic therapy pathway is efficient and open for business. This pathway is a key step in making safe and effective generic drugs available to patients quickly and ensuring there’s adequate competition so patients have affordable access to the treatments they need,” said Gottlieb.
- Washoe County school board approves Renown contract, will explore other health-care options for next year (thenevadaindependent.com)
The Washoe County School District Board of Trustees voted...to renew contracts with Renown Hospital and its insurance arm while also beginning the process of scouting out alternative options over the next year to address the long-term solvency issues of its health insurance fund...trustees voted unanimously to direct the superintendent to begin a competitive request for proposals process over the next year to explore other health-care options for employees and retirees as the district grapples with the rising costs of health care and an insurance fund balance that isn’t keeping up. They then voted to approve three-year contracts with Renown and Hometown Health...Saint Mary’s Health Network, which had held the longstanding contract to provide coverage to district employees, cried foul about the renewal process for the Renown and Hometown Health contracts this year, saying that it could provide health care more cost effectively but hadn’t been given a fair shot to prove to the district that it could do so. Amber Norris, director of business development and marketing for Saint Mary’s, said at the meeting that the request for proposal is “a move in the right direction” and that the health network looks forward to submitting its proposal...
- Moody’s: CMS proposed changes to Medicare’s outpatient prospective payment system could hurt hospitals (healthcarefinancenews.com)
...the Centers for Medicare and Medicaid Services' proposed changes to outpatient services, including site neutral clinic visits, 340B policy changes and broadening the list of surgeries covered at ambulatory surgical centers, would generally be credit negative and hurt hospital margins.
- Changes include no longer paying more for clinic visits in off-campus hospital or provider-based department clinics compared to a physician's office...
- Proposed changes to the 340B policy could also impact certain hospitals...CMS lowered reimbursement for Part B drugs to the drug's average selling price, minus 22.5 percent from the ASP, plus 6 percent. CMS said this would save Medicare about about $1.6 billion...
- CMS has proposed adding some nonsurgical procedures to those covered at ambulatory surgical centers, which are located off-campus, but are not hospital outpatient surgery centers...
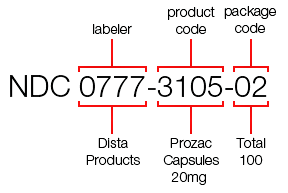
- FDA Anticipates Need for Expanding National Drug Code, Calls for Industry Input (raps.org)
The Food and Drug Administration announced...it plans to hold a public discussion on expanding the National Drug Code format and the impact on industry...FDA currently assigns 10-digit NDCs for the purposes of identifying drugs in the country and the format is comprised of three codes regarding the labeler, the product and the package...“FDA anticipates that it will run out of 5-digit labeler codes in approximately 15 years,” the agency wrote in the notice on the 5 November public hearing. “FDA will begin assigning 6-digit labeler codes at some point in the future due to exhaustion of 5-digit labeler codes.”...Prior to implementing the new formats for longer configurations, FDA officials will seek feedback from meeting participants on the potential impact on companies’ bottom line and drug safety, as well as “issues associated with the current lack of NDC uniformity in the marketplace.”