- Deregulate Pharmacists Now To Increase COVID-19 Vaccine Uptake (reason.com)
Enabling tens of millions of Americans to get themselves speedily vaccinated against COVID-19 will be a huge logistics challenge. A new policy brief from the Mercatus Center, a think tank at George Mason University, argues that we could greatly accelerate the process by removing the complex state regulations that prevent pharmacists from administering vaccines...The...report recommends that state regulators relax age restrictions on pharmacist-administered vaccinations; issue statewide standing orders authorizing pharmacists to administer vaccines without requiring a physician-written prescription for each patient; and revise regulations, as Oregon has, to permit pharmacists to administer all of the vaccines recommended by the CDC's Advisory Committee on Immunization Practices. The latter recommendation "prevents lag in vaccine administration due to boards or legislatures having to approve individually named vaccines for pharmacist administration."...READ MORE
- Pharmacy Board Loosens Restrictions on Hydroxychloroquine Prescriptions, Reversing Course (thetexan.news)Coronavirus: Trump says he’s been taking hydroxychloroquine for a ‘few weeks’ (independent.co.uk)Trump’s use of malaria drug likely to be welcomed in India (apnews.com)Opinion: Hydroxychloroquine at the Center of COVID-19 Discussions (drugtopics.com)Is Big Pharma Suppressing Hydroxychloroquine? (americanthinker.com)
The Texas State Board of Pharmacy issued a new rule that no prescriptions for hydroxychloroquine could be dispensed without a diagnosis, then changed their tune...On March 20...issued a new rule that no prescriptions for hydroxychloroquine or azithromycin could be dispensed without a diagnosis “consistent with evidence for its use.”...Over six weeks after the original rule was published, the Texas State Board of Pharmacy has recently changed its guidance to pharmacists...The website now says, “The rule does not prevent a physician from prescribing one of these drugs for an off-label use. Please note, the intended use for the drug is not required if the practitioner determines the furnishing of this information is not in the best interest of the patient…”READ MORE
- CMS releases new flexibility, waiver protections for providers to help handle coronavirus (fiercehealthcare.com)
The Trump administration has issued blanket waivers and new flexibilities to help hospitals and facilities cope with the coronavirus outbreak...“It is vital that federal requirements designed for periods of relative calm do not hinder measures needed in an emergency,” CMS Administrator Seema Verma said in a statement. “The nationwide waivers we are activating today will be a godsend for those on the frontlines of the fight against this new virus.”...Some of the waivers and flexibilities that CMS took on Friday include:
- Waiving requirements that critical access hospitals limit the number of beds to 25 and length of stay to 96 hours;
- Enabling acute care hospitals to house acute care patients in a separate unit;
- Waiving replacement requirements when durable medical equipment gets damaged or unusable. A contractor can waive requirements such as a new physician’s order, face-to-face requirement and other documentation; and
- Allowing long-term care hospitals to exclude patients stays from the 25-day average length requirement if treatment is required in conjunction with the emergency.
- CMS also sought to quickly approve waivers for states and territories for Medicare, Medicaid and the Children’s Health Insurance Program...READ MORE
- HHS Unveils Strategy to Reduce EHR Burden for Clinicians (healthleadersmedia.com)
The federal government has released its long-awaited plan to reduce red tape and other administration snarls that create time-eating obstacles for doctors using health information technology...The Department of Health and Human Services' Strategy on Reducing Regulatory and Administrative Burden Relating to the Use of Health IT and EHRs, mandated by the 21st Century Cures Act, aims to reduce the effort and time required by clinicians to meet reporting requirements, record health information, and improve the functionality and intuitiveness of EHRs...READ MORE
- Drug payment cuts to 340B hospitals spur debate on best path forward (healthcarefinancenews.com)
Hospitals say revenue from the 340B program is essential, while others contend the original law is being abused...an federal appeals court ruled that 340B hospitals will now be subject to Medicare cuts in outpatient drug payments by nearly 30%... gives...the Department of Health and Human Services the legal authority to reduce payment for Medicare Part B drugs to 340B hospitals...the action means patients – particularly those who live in vulnerable areas – will pay less out-of-pocket for drugs in the Medicare Part B program...providers...said the 340B decision will hurt hospitals and patients in these vulnerable areas...Hospitals that serve large numbers of Medicaid, Medicare and uninsured patients were getting the drugs for a discounted price, but, getting reimbursed at the higher price...The hospitals...are in the red or operating on thin margins, were using the pay gap in the price difference to cover operational expenses. HHS deemed it inappropriate that these facilities would use Medicare to subsidize other activities and initiatives...READ MORE
- CMS Approves More Medicaid Section 1135 Waivers, Bringing Total to 34 States (pharmacytimes.com)
Following the first approved Medicaid section 1135 waiver for Florida, the Centers for Medicare and Medicaid Services has approved 33 more waiver requests in the last 2 weeks....The waivers are intended to provide the states with relief during the coronavirus disease 2019 pandemic by providing states the flexibility to focus resources on managing the outbreak. Some waivers available under Section 1135 of the Social Security Act include temporary suspension of prior authorization requirements; extension of existing authorizations; modified timeline requirements for state fair hearings and appeals; and relaxed provider enrollment requirements to allow states to quickly enroll out-of-state or other new providers...READ MORE
- CAR-T—the Future of Medical Progress Is Now (realclearhealth.com)
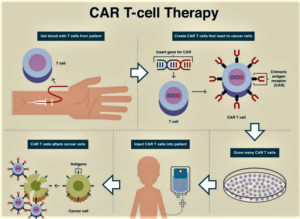
Personalized medicine is the future of medical progress...For instance, an immunotherapy treatment called chimeric antigen receptor T-cell therapy is accessible now, and the Trump administration has the opportunity to make it widely available to Medicare beneficiaries...over 70 Members of Congress—Democrats, Republicans, liberals, and conservatives—sent a letter to Seema Verma, administrator of the Centers for Medicare and Medicaid Services, commending the administration for “ensuring Medicare patients nationwide have access” to this life-saving treatment. The Congressional letter goes on to ask the administration to “ensure that hospitals are appropriately reimbursed so they may continue to provide” CAR-T therapy to America’s seniors...without appropriate reimbursement policy, a Medicare patient could be denied access to a treatment that would save his or her life. Without proper reimbursement by Medicare, providers simply will not be able to offer it as an option, especially in rural areas as patients must stay near a treatment center for four weeks to be monitored...READ MORE
- Rx for anger, as Nellis lockdown bars military retirees from pharmacy (reviewjournal.com)
When retired Navy Chief Petty Officer Richard Gray and his wife, Sheila, were able to go to the Nellis Air Force Base pharmacy, they picked up their prescriptions with full coverage and no copayment...But now their only option is off base, and the 16 medications they take between them cost upward of $2,000 a month out of pocket at Walgreens...Under the base’s public health emergency called on April 3 and renewed May 4 in response to the coronavirus outbreak, only uniformed members and their dependents and essential civilian contract employees are allowed on base, including the pharmacy...The Nellis pharmacy was placed off limits for retirees on April 10, leaving thousands of local military widows and retirees, many of them living on fixed incomes, locked out of the no-cost medication to which they are entitled. Now they are subject to copays that can quickly add up to hundreds of dollars...READ MORE
- OCR will ease restrictions on telehealth tech during COVID-19 (healthcareitnews.com)Trump administration expands Medicare telehealth benefits for COVID-19 fight (healthcareitnews.com)
The HHS Office for Civil Rights announced... that during the coronavirus pandemic it will use discretion when enforcing HIPAA-compliance for telehealth communications tools...Even though some of those technologies may not fully comply with HIPAA requirements, OCR says it "will not impose penalties for noncompliance with the regulatory requirements under the HIPAA Rules against covered health care providers in connection with the good faith provision of telehealth during the COVID-19 nationwide public health emergency."...Covered entities seeking to use audio or video communication tech to reach patients where they live "can use any non-public facing remote communication product that is available to communicate with patients," said the agency. "This exercise of discretion applies to telehealth provided for any reason, regardless of whether the telehealth service is related to the diagnosis and treatment of health conditions related to COVID-19."...READ MORE
- Chinese heparin maker tried to sneak proof of its unapproved APIs out the back door (fiercepharma.com)
On arrival for a pre-approval inspection for a Chinese heparin API maker, an FDA inspector was told that the plant had not started making products and was only doing equipment testing. The truth of the matter was hidden in a drum being snuck out the back. An intercepted container an employee was removing from the warehouse contained two batches of crude heparin manufactured two days earlier...That was just the beginning of the problems found during July 2019 inspection of Yibin Lihao Bio-technical, the API maker in Yibin Shi Sichuan, China. According to a warning letter, there were quality assurance records scattered in cabinets, on desks and on the floor of the QA office. Employees insisted they were for a grant application from the government and not manufactured products. Not true. The FDA would learn the records did correspond to manufactured heparin...READ MORE