- FDA Begins Adding Suffixes to Newly Approved Biologics’ Names (raps.org)
The US Food and Drug Administration this week began adding four-letter meaningless suffixes at the end of newly approved biologics' nonproprietary names, signaling a shift in policy from only adding the suffixes to biosimilars' nonproprietary names since 2015...The first additions of the meaningless suffixes came for...approval of Roche's Hemlibra (emicizumab-kxwh), one of the first new medicines in nearly two decades to treat people with hemophilia A, and...approval of Ultragenyx Pharmaceutical's Mepsevii (vestronidase alfa-vjbk) to treat pediatric and adult patients with a rare inherited condition called mucopolysaccharidosis type VII (MPS VII), also known as Sly syndrome...The newly added suffixes were not preceded by an announcement from FDA, though the shift was not entirely unexpected...Back in January, FDA finalized guidance on how biosimilars and their biologic reference products' names should include this four-letter, FDA-designated meaningless suffix attached at the end of the nonproprietary name...But until this week, only new biosimilars had the suffixes attached to their names. The agency did not respond to a request for comment on why new biologics' nonproprietary names included the suffixes this week.
- Hospitals file lawsuit to block 340B drug payment cut (biopharmadive.com)
The American Hospitals Association and other organizations have made good on their promise to sue the federal government in an effort to halt a major cut planned for the 340B Drug Pricing Program...The hospitals are arguing the reimbursement change exceeds the authority of the HHS secretary and is "arbitrary and capricious." They ask the court to force HHS to delay implementation or strike the cut entirely...HHS released a final rule earlier this month that changes the amount 340B hospitals will be paid for most drugs to 22.5% less than the average sales price starting at the beginning of next year. Currently those hospitals are paid the average price plus 6%...The cut of about 30% would hurt nonprofit hospitals' margins, according to a recent report from Moody's. The CMS has argued the change would reduce out-of-pocket costs and improve patient-provider relationships. The agency has calculated the 340B cut would save about $900 million next year...AHA has been strongly critical of the cut, saying it threatens patient care, particularly for vulnerable populations that are most likely to use safety net hospitals benefiting from the drug payment program...A recent report found 340B hospitals had a larger decrease in charity care spending than other hospitals, but AHA said the study was misleading and incomplete.
- Drug bingo: EU readies vote for London-based pharma agency (reuters.com)'Hard' Brexit risks disrupting supply of thousands of drugs (biopharmadive.com)
Nineteen EU governments will slug it out in a series of secret ballots in 10 days’ time for the right to host the European Medicines Agency once it leaves London after Brexit...Confirming that none of the cities, from Porto to Helsinki, has dropped out of the running...those organizing it confessed they had no idea how long the voting would take...The EMA itself has warned that moving to some of the cities on the list -- sources have said notably those like Warsaw or Sofia in eastern Europe -- would see so many of its 900 London staff quit that it would harm Europe’s health. Top choices for employees are Amsterdam, Vienna and Barcelona...States agreed to a number of criteria, including that cities chosen should offer premises ready for a start in March 2019, when Britain leaves the EU, be accessible from across Europe and take account of “geographical spread” -- the fact newer members in the east host fewer agencies than richer neighbors.
- CMS Shifts Coding and Payment Policy for Biosimilars Under Medicare Part B (raps.org)
The Centers for Medicare and Medicaid Services...announced that it would finalize a policy to separately code and pay for biosimilar products under Medicare Part B, signaling a win for industry...CMS said it is making the change as it was "persuaded that that there is a program need for assigning Part B biosimilar biological products into separate HCPCS [Healthcare Common Procedure Coding System] codes, specifically that this policy change will address concerns about a stronger marketplace, access to these drugs in the United States marketplace, provider and patient choice and competition."...industry groups and companies urged CMS to revise its biosimilar reimbursement policy to provide for separate HCPCS codes for each biosimilar and to reimburse each biosimilar based on its own average sale price..."Effective January 1, 2018, newly approved biosimilar biological products with a common reference product will no longer be grouped into the same HCPCS code. We will issue detailed guidance on coding, including instructions for new codes for biosimilars that are currently grouped into a common payment code and the use of modifiers....
- Ohio’s PDMP Gives Pharmacists Better Patient View (drugtopics.modernmedicine.com)
After a successful pilot program with Kroger’s pharmacies, Ohio’s prescription drug monitoring program is the first in the United States to offer advanced analytics for pharmacists and health care providers...The State of Ohio Board of Pharmacy is working with Appriss Health, provider of a comprehensive platform for substance use disorder, to enhance the state’s PDMP, known as Ohio Automated Rx Reporting System...The PDMP now provides NarxCare, an advanced analytics and patient support solution, to Ohio prescribers and pharmacists, in clinical workflow and via OARRS, to assist in clinical decision-making and promote patient safety. The analytics are available for free to Ohio healthcare providers and pharmacists accessing OARRS via electronic health records and pharmacy management systems...NarxCare...aggregates and analyzes prescription information from providers and pharmacies...then presents visual, interactive information — as well as advanced analytic insights, complex risk scores...The system also provides tools and resources that support patients’ needs and connects them to treatment, if necessary.
- US transportation workers to face testing for prescription opioids next year (cnbc.com)
Safety-sensitive transportation workers — including flight crew, air traffic controllers, truck drivers and train engineers — will be screened for several common opioid painkillers starting next year, according to a new federal rule...Previous drug tests screened for cocaine and marijuana. The rule adds screening for hydrocodone, hydromorphone, oxymorphone and oxycodone...The broadened testing will take effect Jan. 1, 2018..."The opioid crisis is a threat to public safety when it involves safety-sensitive employees involved in the operation of any kind of vehicle or transport," Transportation Secretary Elaine Chao said in a release. "The ability to test for a broader range of opioids will advance transportation safety significantly and provide another deterrence to opioid abuse, which will better protect the public and ultimately save lives."
- FDA commissioner warns drug companies of ‘disruptive’ regulations to fight opioid epidemic (cnbc.com)
The Food and Drug Administration is likely to take new actions on opioids that may be "disruptive" and "uncomfortable" to drugmakers, the agency's commissioner (Scott Gottlieb) said...In addition to seeking to treat opioid-addicted patients with alternative medications that don't produce a high, the FDA says it will look at ways to reduce exposure to the drug. That includes new ways of packaging and distribution..."For example, it's possible that a defined, short-term supply of medication could be packaged in a manner that limits the number of pills dispensed,"..."We're at a point in this crisis that we're going to have to think of ideas and taking actions that are going to be more disruptive and are going to be uncomfortable to some parties," Gottlieb told "Squawk Box." "But we have to take more vigorous action to get ahead of this."...Gottlieb said the agency is having discussions with drug companies about the new packaging solutions..."Something like this could move potentially quickly," he said. "We're invested in taking a hard look at this and seeing what the opportunities are."...
- Health exchange eyes return to state-run enrollment site (lasvegassun.com)
Nevada’s health exchange is looking to once again run its own enrollment site, a move off the healthcare.gov platform that could save the state millions of dollars...The Silver State Health Exchange is planning to issue a request for information in December and will likely issue a request for proposal in March, said Heather Korbulic, the agency’s executive director. She said the healthcare.gov platform limits the exchange’s access to information and how much time customers can spend shopping for plans...“We’ll just take the same business processes that we use with healthcare.gov right now and remove healthcare.gov and put in private technology,” she said. “So we limit any kind of disruption.”...She said the exchange can show that it has a plan that will comply with regulations while still going to a private option. If the new platform is in place by 2020, the exchange would save $6 million...
- DEA cracking down on fake fentanyl traffickers (statnews.com)
The U.S. Drug Enforcement Administration wants to make it easier for federal prosecutors to go after people who peddle illicit versions of the deadly opioid fentanyl that are fueling the nation’s drug abuse crisis...The agency said Thursday it intends to classify drugs that are chemically similar to fentanyl as illegal controlled substances, letting prosecutors avoid the hurdles they often face in bringing charges in such cases...The move aims to stop the flow of fentanyl variants into the U.S. as the opioid abuse crisis rages...The new classification, which would last two years, lets prosecutors bring charges against fake-fentanyl traffickers under the federal Controlled Substances Act. Prosecutors have been able to use the Analogue Act for cases involving variants, but that requires them to call chemists and other experts to prove that, while molecularly different, the drugs are just as potent and dangerous as fentanyl in its true form. Prosecutors often complain of being stymied by the additional hurdles that delay cases and sometimes lead to charges being dropped...Law enforcement officials are frustrated by chemists who frequently alter the chemical compounds in their drugs to create substances that are not expressly illegal. Justice Department and DEA officials say they play a constant game of “whack-a-mole” to keep up with the changes.
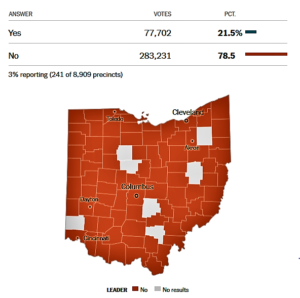
- Ohio Ballot Issue 2: Cap State Agency Drug Costs (nytimes.com)Handing pharma a win, Ohio voters overwhelmingly reject drug pricing measure (fiercepharma.com)
An initiative on Tuesday’s ballot in Ohio is aimed at reducing the cost of prescription drugs in the state. The measure would cap the price of prescription drugs purchased by the state government, including Medicaid...The measure drew strong resistance from drug makers, which spent more than $49 million to try to kill it, and the industry is not accustomed to losing political fights. Last year, it successfully killed a measure in California that was similar to the one in Ohio, but only after spending more than $100 million to do so.