- U.K. weighs drug rationing as NHS England’s budget tightens: report (fiercepharma.com)
Patients in the U.K. face yet another barrier to access as a tough budget situation has forced the country’s healthcare system to consider rationing costly drugs...Beginning in April, cancer patients and others could have to line up for medicines that cost NHS England more than £20 million per year...That’s even after those meds have been deemed cost-effective by the National Institute for Care and Excellence...The country’s pharmaceutical association, ABPI (Association of the British Pharmaceutical Industry) pointed out some medicines that could see rationing…The agency (NHS) determined that, based on NICE appraisals between April 2015 and July 2016, 81% of medicines would fall under the £20 million a year “budget impact threshold” for a total spend of £125 million. The 19% of drugs above the threshold had a budget impact of nearly £400 million...This is about introducing cost effective, but expensive treatments onto the NHS in a way that does not compromise its ability to fund other areas of work...
- U.S. Supreme Court agrees to hear dispute over biologic drug sales (reuters.com)
The U.S. Supreme Court...agreed to hear a dispute over whether companies that make copycat versions of biologic drugs must wait six months after winning federal approval to begin selling them...an appeal by Novartis AG of a 2015 federal appeals court decision that prevented the...company from selling its biosimilar version of...Amgen Inc's $1-billion-a-year Neupogen until six months after the Food and Drug Administration approved it. The case could determine how quickly patients have access to biosimilar medicines at potentially cheaper prices...The dispute arose when Amgen sued Sandoz...alleging patent infringement and violations of the law governing biosimilars. The companies disagreed on how to apply the law's requirement that a biosimilar drug maker give the brand-name manufacturer 180 days notice before launching its copycat version...The justices...also agreed to resolve Amgen's appeal in the same case over whether biosimilar makers must give brand-name manufacturers a copy of their application to make a copycat drug after it is submitted to the FDA.
- Nevada State Board of Pharmacy – Newsletter January 2017 (bop.nv.gov)
- Changing Faces
- Bowl of Hygeia Awarded to Adam Porath, PharmD
- No Prescription Needed!
- FDA Issues Final Rule Amending List of Drug Products That May Not Be Compounded
- Selected Medication Risks to Manage in 2017
- Environmental Factors, Workflow, and Staffing Patterns – Poor Quality Lighting
- DEA to Decrease Manufacturing Amount of Opioid Controlled Substances in 2017
- New CDC Brochure Offers Pharmacists Tips for Addressing Prescription Opioid Abuse and Overdose
- FDA Requires Boxed Warnings and Patient Focused Medication Guides Indicating Serious Risks Related to Combined Use of Certain Opioid Medications and Benzodiazepines
- FDA’s Division of Drug Information Offers CE Webinars for Students and Clinicians
- FDA Approves Labeling Changes for All Prescription Testosterone Products
- Latest FDA Drug Info Rounds Training Videos Available
- Inspector's Corner
- Ghost Towns and Medicines
- Scheduling data altered at VA’s Las Vegas-area mental health clinics, report says (reviewjournal.com)
Schedulers for VA mental health services in the Las Vegas area altered wait time data in 2013, months before a national scandal erupted over similar practices at a VA hospital in Arizona, according to a long-delayed report by the agency’s inspector general...A report summary by the Veterans Administration’s Office of the Inspector General, published this week, did not conclude that the practices were a deliberate attempt to fool a tracking system of wait times...But it noted that “several of the (medical support assistants) interviewed indicated that they were directed by supervisors to manipulate scheduling data.”…In response to the report, the VA Southern Nevada Healthcare System...disputed the allegation that the wait times were deliberately manipulated to fool the tracking system, saying the Inspector General’s Office found no hard evidence to support the claims...Rep. Dina Titus...released a statement...calling the allegations in the initial complaint troubling and requesting a briefing on steps taken to ensure the problems have been resolved...“I have been assured for years that this was not happening in our state,” Titus said...“Such behavior is shameful and unacceptable.”
- The FDA targeted DTC, video, unapproved drug promotion in 2016 (mmm-online.com)
The FDA is taking a closer look at how drugmakers use channels like direct-to-consumer advertising and video, a shift that has raised questions for pharmaceutical marketers...On its face, the FDA's Office of Prescription Drug Promotion's batch of enforcement letters in 2016 seems par for the course, with the agency issuing a total of 11 letters, compared to 2014's and 2015's totals of nine each. But these letters contain important warnings for drugmakers about the FDA's evolving views on promotion, and what channels, tactics, and drugs it's examining closely…The OPDP...issued two enforcement letters for DTC TV ads developed by Sanofi for its insulin Toujeo and Celgene for its psoriasis drug Otezla. Regulators said the ads were distracting to viewers because they used frequent scene changes — and, in Otezla's case, abrupt changes in music — to distract viewers from presented risk information...These untitled letters were noteworthy because they signal a subtle shift in enforcement actions, with the FDA now examining tactics within DTC ads rather than focusing on bigger, more serious infractions, such as omitting risk information entirely…the FDA saying the viewer couldn't understand it because the imagery was too fast moving or the music was too catchy in the background...
- Non-Proprietary Naming of Biologics and Biosimilars: FDA Finalizes Guidance (raps.org)
In a departure from the way the WHO and Europe name biologics, the US Food and Drug Administration...finalized long-awaited guidance on how biosimilars and their biologic reference products’ names should include a four-letter, FDA-designated meaningless suffix attached at the end of the nonproprietary name...The decision to finalize this guidance follows...but large opposition to the idea of using meaningless rather than meaningful suffixes that could make it easier to distinguish the manufacturers of the products...For example...Sandoz’s Zarxio, which includes a non-proprietary name with a meaningful suffix (Sndz for Sandoz): filgrastim-sndz...But FDA has said it will change Zarxio’s nonproprietary name from filgrastim-sndz to “filgrastim-bflm." And Amgen's Neupogen (filgrastim) would be changed to "filgrastim-jcwp."...But these suffix rules do not necessarily apply to all related biologics...some instances it has designated a proper name that includes an identifier attached as a prefix to distinguish the products from previously licensed biologics. For example, with ado-trastuzumab emtansine, FDA includes a unique prefix, which it says was necessary to minimize certain medication errors and to facilitate pharmacovigilance.
- Nevada leaders want Heller to explain how key parts of ACA will be replaced if measure is repealed (reviewjournal.com)
The top two Democrats in the Nevada Legislature are calling on U.S. Sen. Dean Heller to explain how Republicans in Congress will replace key provisions of the Affordable Care Act if they repeal it...Aaron Ford and Jason Frierson...sent a letter...posing...questions to Nevada’s senior senator in Congress...the state leaders asked what steps will be taken to ensure people who purchased coverage through the state exchange and those who receive federal tax credits will still be able to buy affordable insurance...what will be done to ensure more than 217,000 Nevadans receiving coverage under expanded Medicaid eligibility will remain covered; guarantees for women’s health care; and continued mandates to cover people with pre-existing conditions and allow parents to keep children on their policies until age 26...“I would anticipate nobody’s going to lose their health care for the next two or three years until the replacement is put in place,” Heller said...
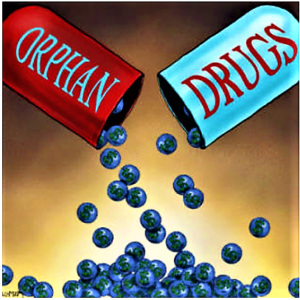
- Drugmakers ‘hijacked’ the FDA’s orphan system to score premium pricing on mass-market meds: report
There’s no denying that financial incentives for orphan drug development spawned meds that have saved hundreds of thousands of lives. But they’ve also helped mass-market drugmakers rack up millions in incentives, tax breaks and patent-protected profits—in some cases through monopoly pricing...About one-third of the orphan drug approvals the FDA doled out since the program began more than 30 years ago have been for repurposed, large-market products or drugs with multiple orphan green lights...Best-sellers such as Crestor…,Abilify…,Herceptin…,and Humira...fall into the category of big sellers whose makers snagged millions in government incentives—not to mention seven years of exclusive rights on the market—when they resubmitted their therapies as treatments for smaller populations...What we are seeing is a system that was created with good intent being hijacked…Repurposing a drug isn’t necessarily a bad thing, of course, if it can help get a treatment to additional patients...But when the orphan incentives allow competition-free drugmakers to charge whatever prices they want for their meds?...Now...it seems like...this practice may be driving up prices...Industry lobby groups...are unsurprisingly in favor of maintaining the status quo. With rare diseases “tragically killing and brutalizing mostly children,” incentives for orphan drugmakers should be kept in place...the risk of losing incentives in the system far outweighs the benefit of trying to save a few pennies on the health care dollar...
- FDA warning letter tells Clorox to clean up Aplicare plant (fiercepharma.com)
The FDA says the unit of Clorox that manufactures povidone-iodine drug products needs to clean a big mess at a plant in Meriden, Connecticut, which failed to follow steps to insure the sterility of its wound products—products that the FDA also said are “unapproved.”...The agency...posted a warning letter for the Aplicare plant…(it) said...the plant failed to implement adequate microbial controls...some of its products are unapproved because they do not comply with the FDA’s OTC Final Monograph for Topical Antifungal Drug Products.
- FDA Warns Wockhardt for Destroying CGMP Documents, Other Violations (raps.org)
...Indian drug manufacturer Wockhardt is in trouble again with the US Food and Drug Administration, this time for destroying current good manufacturing practice documents, among a list of other major violations...FDA sent a warning letter to the company following an eight-day inspection...of its Ankleshwar...manufacturing site that uncovered “torn and shredded equipment maintenance documents, raw material labels, and change control work orders” in the site’s scrap yard awaiting incineration...The site was banned from shipping products to the US in August...This is the latest in a series of failures by Wockhardt to meet the standards of not just FDA but other regulators...