- CMS finalizes plan to boost wage index for rural hospitals (fiercehealthcare.com)
The Trump administration released a final rule...that boosts Medicare payments to rural hospitals in a bid to address major inequalities...The rules included an increase to the wage index for certain low-wage hospitals such as those in rural areas..."The changes we’re finalizing in today’s rule are long overdue and improve the way Medicare pays hospitals, which will help many rural hospitals maintain their healthcare labor force," CMS Administrator Seema Verma...READ MORE
- Putting safety first: Retailers wrestle with supply chain safety amid drug import push (drugstorenews.com)
Product safety is a multifaceted issue for any retailer dealing with the pharmacy and prescriptions, and the systems that are in place to protect the quality and integrity of the goods offered constantly are evolving to strengthen the industry’s defenses...One of the key loopholes between consumers and the dangers of unsafe products, however, is the potential for the introduction of items from other countries that fail to meet the rigorous safety standards of the United States...The Food Safety Modernization Act, which took effect in 2011, sought to close that gap by imposing tighter regulation around the import of foods and ingredients from overseas...Market pressures in the pharmaceutical industry, however, have led officials at both the state and federal levels to explore the possibility of bringing drugs in from other countries at a lower cost, potentially circumventing the Food and Drug Administration’s close oversight of U.S.-made medications...the...National Association of Chain Drug Stores see several potential problems with the import of prescription medications from other countries, including:
Issues around the adequacy, consistency and integrity of the supply;
Issues around quality and safety;
Problems with maintaining dual inventories inside pharmacies;
Burdensome testing requirements;
Costs of establishing an infrastructure for imported drugs; and
Liabilities for injuries caused by imported drugs...READ MORE
- Albertsons pharmacy technicians first in Idaho trained to prescribe naloxone (drugstorenews.com)
As a new Idaho law allowing pharmacy technicians to prescribe naloxone takes effect, Albertsons announced that its Boise-area pharmacy technicians are the first in the state who have been trained to do so...The law, signed in February, makes Idaho the first state in the nation to add pharmacy technicians to the ranks of pharmacists, physicians and nurse practitioners in being able to prescribe the opioid overdose reversal drug. Idaho Gov. Brad Little also issued an executive order creating an advisory group in the state to guide healthcare decisions and strategies related to opioid misuse...“Idaho is leading other states in the steps we are taking to expand access to this critical medication and in our efforts to address the opioid crisis,” Little said. “Training pharmacy technicians and all health professionals to prescribe naloxone further reaches our underserved and rural communities. Albertsons has been a key partner in increasing the scope of practice for both pharmacists and technicians to continue improving access to beneficial and lifesaving medications.”...READ MORE
- NACDS applauds new Texas law to help curb opioid abuse (drugstorenews.com)
The National Association of Chain Drug Stores today praised Texas’ enactment of a bill (HB 2174) to address opioid abuse and addiction, while providing key safeguards to address the needs of those suffering from chronic pain...the new law will require electronic prescribing for controlled substances, to help prevent fraud and abuse. It also will limit the supply of a patient’s first opioid prescription to 10 days, when that prescription is for temporary, or acute, pain. It is important to note that this limit does not apply to prescriptions for ongoing, or chronic, pain. It also does not apply to cancer care, treatment of other illnesses, or end-of-life care...READ MORE
- Trump firms up plan to import medicines; pharma companies resist (reuters.com)
The Trump administration took a step...toward allowing importation of medicines from Canada, an action the president has advocated as a way to bring cheaper prescription drugs to Americans, but the pharmaceutical industry was quick to resist the move...The U.S. Department of Health and Human Services said it and the Food and Drug Administration will propose a rule that will allow it to authorize states and other groups to pursue pilot projects related to importing drugs from Canada...READ MORE
- Resort corridor hospital targeted by new Nevada law seeks to dispel misconceptions (lasvegassun.com)
A year after its opening and a few months after it was targeted in a political debate in the Nevada Legislature, Elite Medical Center...is looking toward 2021...That’s the year that Elite will have to accept Medicare and Medicaid, although CEO Patty Holden, who said it could be much sooner, hopes to work with lawmakers to create different classes of hospitals in Nevada...Less than a mile from Strip landmarks like the Bellagio and Paris Las Vegas, the hospital is working toward accepting Medicare and Medicaid, after state legislators earlier this year passed a law requiring essentially all hospitals in the state to accept the government programs...READ MORE
- FDA’s overreach will harm compounding pharmacies and the patients they serve (statnews.com)
The deaths of 64 people and sickening of nearly 800 due to criminal negligence by employees of the New England Compounding Center in 2012 marked a profound failure of state and federal regulatory enforcement…led to…the Drug Quality and Security Act of 2013 provisions to create a more robust regulatory framework for compounding pharmacies…The legislation instructed the FDA to create regulations…that would assure patient safety while permitting local compounders to continue to meet patient needs by providing customized compounded medications using FDA-approved substances…But the FDA has overreached in implementing the provisions, all but halting common compounding practices that have been safely performed for years and on which patients with legitimate needs for compounded medications rely. Not only that, but the FDA has done so by circumventing the federal Administrative Procedure Act, issuing “guidance documents” to implement policies rather than following the statutory rule-making process that requires stakeholder input regarding proposed regulations…READ MORE
- Our STAT Op-Ed: Drug Importation Can’t Coexist with U.S. Track-and-Trace Law (drugchannels.net)
STAT recently published our op-ed: State drug importation laws undermine the process that keeps our supply chain safe...there is no legal or operational way of transforming a drug packaged for a foreign market into a drug that meets the U.S. requirements of our in-progress track-and-trace system. What’s more, there is no way to alter the law to enable importation without undermining the law’s purpose and value...States can't wish away the requirements of a significant federal law. Either we have a secure drug supply chain or we don’t...READ MORE
- Why the U.S. is building a track-and-trace system
- How track-and-trace works
- Importation undermines the track-and-trace system
- Importation enables counterfeits
- Forgetting the lessons
- Nevada State Board of Pharmacy July 2019 Newsletter (bop.nv.gov)
- CS: Reporting Theft or Loss
- National Pharmacy Compliance News July 2019
- FDA Changes Opioid Labeling to Give Providers Better Information on Tapering
- DEA Warns of Scam Calls Targeting Pharmacists and Other DEA-Registered Providers
- FDA Officials Outline 2019 Efforts to Improve Quality of Compounded Drugs
- China Agrees to Stricter Fentanyl Production Laws Following Pressure From US Lawmakers
- Two Lots of Transdermal Fentanyl Patches Recalled Due to Product Mislabeling
- FDA Releases Toolkit to Help Promote Safe Opioid Disposal
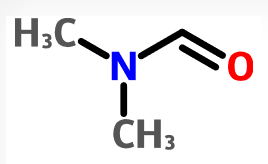
- Pharmacy takes FDA to task in citizen petition over tainted valsartan (fiercepharma.com)
The FDA has tied the contamination of blood pressure drugs by probable carcinogens to an approved switch in manufacturing to a process that uses certain solvents. Now an online pharmacy says it has discovered the solvents are as bad as the impurities they have been creating and has criticized the FDA for not taking steps to limit their use...In a citizen petition...Valisure says the FDA has established acceptable limits for impurities such as those commonly known as NDMA and NDEA after they were discovered in heart drugs last year, leading to massive global recalls...But it claims the FDA has not lowered the acceptable level for the solvent DMF, although it “has become apparent that the switch in the manufacturing industry to the use of the DMF solvent may be largely responsible” for the appearance of the impurities in the U.S. drug supply...The petition says the FDA needs to investigate the use of the solvent N,N-Dimethylformamide in drug manufacturing since it is classified as a Class 2 carcinogen...READ MORE