- FDA faulted for failure to track safety issues with drugs already on market (statnews.com)
Most Americans assume that drugs approved by the Food and Drug Administration are safe to take as directed. But safety concerns often arise only after the drugs go on the market, when companies or doctors tell the FDA about cases of patients who have fallen ill or died from their medications...a federal watchdog agency said the FDA is failing to sufficiently track and publicly disclose instances in such cases...Government Accountability Office investigation...raises...concerns about the FDA’s oversight. It expresses particular concern about the lack of tracking of drugs cleared under two expedited approval programs, which account for about one-quarter of all medicines permitted to go on the market...investigators also criticized the FDA for failing to post quarterly reports listing certain potential safety issues that it has identified. Despite a statutory requirement that it do so, last year FDA posted no reports at all in its tracking system...FDA lacks fundamental resources and leadership in ensuring that drugs brought quickly to market are truly safe and effective,”...“If FDA is shifting more of the safety risk to consumers by allowing fewer and shorter clinical trials on expedited drugs, adequate tracking of drug safety issues and review of post market studies are absolutely vital.”...The backlog of postmarket data has been a recurring problem at the FDA...
- Nevada Health Link records 77,411 enrollees, topping previous enrollment period (reviewjournal.com)
Two days ahead of a key signup deadline, the federal government released new enrollment numbers for Nevada's health insurance exchange...Nevada Health Link had 77,411 enrollees on Wednesday, the Department of Health and Human Services reported...That means the exchange has already bested the 72,000 enrollees that bought coverage through the marketplace in all of 2015's open-enrollment period...The department said the latest numbers include existing customers who were automatically re-enrolled, though officials didn't break down how many enrollees were new versus returning...Consumers who haven't bought coverage by Jan. 31 face a federal tax of 2.5 percent of household income, or $695 per adult and $347.50 per child up to a household maximum of $2,085 — whichever is higher.
- Providers slam CMS proposal meant to curb drug abuse (modernhealthcare.com)
Providers are urging the CMS to drop a proposal aimed at combating prescription drug abuse. They say mandating a review of state-run drug prescription databases could lead to inaccurate information and would be an administrative burden for them. They also say frequent prescription changes might reveal a lack of coordination among providers, not drug abuse...As part of a proposed rule on changes to discharge plans, the CMS suggested mandating that providers consult a patient's history on their respective state's prescription-drug monitoring program. The goal is to identify a patient's risk of nonmedical use of controlled substances by tracking substance-use disorders...“Requiring their use for all patients would overwhelm current systems and make it impossible for them to focus on the true at-risk groups they are intended to serve”...the required use of the PDMP for all patients will slow down the discharge process unnecessarily without adding value for patient care...Under the proposed rule, first announced in a November overhaul of the discharge process for hospitals, rehabilitation facilities and home health agencies, providers would be required to develop a discharge plan within 24 hours of a patient's admission or registration. They would have to complete that plan before the patient is discharged.
- NIH asked to fight price gouging by overriding drug patents (statnews.com)
A group of 50 congressional lawmakers wants the Obama administration to develop guidelines that would require drug makers to license their patents to others in a bid to end “price gouging.”...In their letter, they argue that the National Institutes of Health has the ability to issue so-called march-in rights, which refer to overriding a patent. Under federal law, this allows an agency that funds private research to require a drug maker to license its patent to another party in order to “alleviate health and safety needs which are not being reasonably satisfied” or when the benefits of a drug are not available on “reasonable terms.”...the lawmakers argue that “reasonable guidelines can discourage price gouging.” The letter was released...by the Affordable Drug Pricing Task Force...their reasoning, the lawmakers emphasized that march-in rights should only be used when “wrongdoing occurs” and that “innovation should not be threatened.” By issuing guidelines, they argue the NIH would help drug makers make “better-informed pricing decisions.”
- Compounding Pharmacy Forced to Stop Production Due to Insanitary Conditions (specialtypharmacytimes.com)Federal judge enters consent decree against Downing Labs (fda.gov)FDA sues to stop a wayward drug compounder (statnews.com)
Compounding pharmacy Downing Labs LLC (formerly known as NuVision Pharmacy), its co-owners, and its pharmacist-in-charge have been issued a consent decree of permanent injunction...The Texas-based company is allegedly in violation of current good manufacturing practice requirements under the Federal Food, Drug, and Cosmetic Act...Downing Labs is accused of manufacturing and distributing adulterated drugs that were made in insanitary conditions, meaning they were bad enough to endanger public health...“Despite multiple warnings to the company, Downing Labs continued to manufacture injectable drugs under insanitary conditions, putting the health and safety of patients at risk,”...“The FDA pursued appropriate and aggressive action to protect the public health.”...Downing Labs said it has worked "collaboratively and cooperatively” with the FDA to reach an agreement that will enable it to resume the production of compounded sterile medication...also noted that, as part of the consent decree, it voluntarily agreed to participate in a regular program of testing, audit, and inspection “to ensure it is achieving and exceeding its quality goals.”
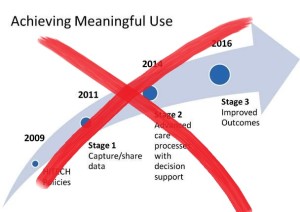
- CIOs celebrate end to meaningful use, want more details on future programs (healthcareitnews.com)Andy Slavitt puts meaningful use on ice; Read his J.P. Morgan speech transcript (healthcareitnews.com)
Some execs say easing off of the requirements will allow providers to focus more on innovation...Healthcare chief information officers breathed a sigh of relief on Tuesday when Andy Slavitt said the end of the meaningful use program was near. But many are waiting on the details before celebrating too much...The acting administrator of the Centers for Medicare and Medicaid Services Andy Slavitt said in a speech at the J.P. Morgan Healthcare Conference that meaningful use would be replaced with a more streamlined regulatory approach in line with the merit-based alternative payment models of the Medicare Access and CHIP Reauthorization Act of 2015...“The early stages of meaningful use took the country from ‘zero to 60' in five years, a remarkable achievement that would not have been possible without HITECH,”...“Now, it's time for the new payment model that rewards providers for achieving better health outcomes to be the driver of innovation, using the new electronic infrastructure that is now in place. That was the vision for ‘Phase 3’ from the very beginning.”..."It's truly unfortunate, but I'm not surprised,”...“The lack of alignment in Washington, varied interpretations by our industry and its vendors, and the resulting and inefficient ‘MU clicks,’ as termed and borne by our physicians have led down this path."...“The sad part of it all is that MU was designed with best intentions yet fated by regulation and political interests,”...
- English doctors strike for first time in 40 years (reuters.com)Industrial action: junior doctors provide emergency-only care (bma.org.uk)
English doctors staged their first strike in 40 years on Tuesday over government plans to reform pay and conditions for working anti-social hours, in a move health chiefs have warned could put patients' lives at risk...Junior doctors, or doctors in training, who represent just over half of all doctors in the state-funded National Health Service, said they would only deliver emergency care during the 24-hour walkout..."This strike is not necessary, it will be damaging," Prime Minister David Cameron said on Monday. "We will do everything we can to mitigate its effects but you cannot have a strike on this scale in our NHS without real difficulties for patients and potentially worse."...Most people in England are supportive of the strikes, as long as emergency care is still provided...The doctors' union the British Medical Association said the contract does not provide proper safeguards against doctors working dangerously long hours.
- Drug Diversion in the 340B Program (pharmacytimes.com)
Section 340B of the Public Health Service Act requires drug manufacturers participating in the Medicaid Drug Rebate Program to sign an agreement...This...limits the price that manufacturers may charge certain covered entities for covered outpatient drugs...Drug diversion in the program is defined as a 340B drug being provided to an individual who is not an eligible outpatient of that entity and/or dispensed in an area of a larger facility that is not eligible (eg, an inpatient service or a non-covered clinic)...in 2013 that 94 audits were underway, which included 700 outpatient facilities and 1930 contract pharmacies. During these audits, drug diversion, duplicate discounts, and ineligible sites/providers were the common areas of noncompliance... Noncompliance to 340B program impacts patients’ bottom line because the more diversion that occurs, the more drug manufacturers increase prices for both public and private insurers, leading to an increase in rates and charges to patients. If HRSA were able to enforce 340B regulations and audit all hospitals on a continual basis, there would be fewer cases surrounding duplicate discounting, drug diversion, and ineligible site/providers...The 340B Program is in desperate need of stronger controls and more audits. Through proactive monitoring of drug inventory and dispensing, 340B drug diversion would decrease, leading to a decrease in drug spending.
- FDA castigates China’s Zhejiang Hisun for ‘systemic data manipulation’ (fiercepharmamanufacturing.com)
A Chinese drugmaker that has a joint venture with Pfizer to produce generic drugs and plans to separately make biosimilars, has been savaged by the FDA in a warning letter for serious data manipulation and shipping to the U.S. products that repeatedly failed follow-up testing by customers...A warning letter posted by the FDA today shows why the agency last September decided to ban products coming out of the Zhejiang Hisun Pharmaceutical plant in Taizhou City. "We observed systemic data manipulation across your facility, including actions taken by multiple analysts, on multiple pieces of testing equipment, and for multiple drugs,"...its concerns over data manipulation were "heightened by the significant number of customer complaints for subpotency and out-of-specification impurity levels the company received from 2012-2014." There were more than 60 complaints for impurity problems...The FDA said the explanations that Zhejiang Hisun Pharmaceutical gave for why data was deleted and raw data was no longer available were completely inadequate, as were the responses to other issues. It said it will continue to prevent the company's products from coming into the U.S. until the agency is satisfied with the steps the drugmaker has taken to bring up its manufacturing standards and can convince the FDA that its test data is authentic.
- How much are junior doctors paid, and why are they threatening to strike? (telegraph.co.uk)Junior doctors row: David Cameron asks doctors to call off strike (bbc.co.uk)
As the British Medical Association prepares for industrial action, ending in the first 'all out' strike by medics in the history of the NHS, we examine the issues...Junior doctors are on the verge of their first day of industrial action, marking the first of three days that ends next month with the first "all out" strike by medics in the history of the NHS...Under the plans, junior doctors will provide "emergency care only" action for Tuesday 12 January, and Tuesday 26 January, followed by a full walk out from 8am to 5pm on Wednesday 10 February...What's caused such ire in the medical community? Is it all about pay? How justified is their anger? Read on for all you need to know.
- Why are they so cross?
- What do the current proposals look like?
- What will strike action involve?
- Is the dispute all due to the 7-day NHS idea?
- Why won't the two sides talk?
- Weren't doctors meant to strike in December?
- How much do junior doctors get paid?
- How does this compare to other starting salaries?
- But what about cuts to their pay?
- Would doctors be put off certain specialities?
- How will their pay supplements change?
- What about working hours?
- When would these new contracts come into force?
- What happens next?