- Better insurance coverage of non-drug therapies might help ease opioid crisis (reuters.com)Coverage of Nonpharmacologic Treatments for Low Back Pain Among US Public and Private Insurers (jamanetwork.com)
When it comes to non-drug therapies for back pain, U.S. insurance plans vary widely in what they will cover, a new study finds...Private and public insurers are missing important opportunities to promote alternatives to opioids...insurers often provide little or no coverage for evidence-backed interventions for chronic pain such as acupuncture and psychological counseling...“Insurers can be part of the problem or part of the solution,” said study coauthor Dr. Caleb Alexander...at the Center for Drug Safety and Effectiveness...“All too often doctors reach for the quick solution, prescription drugs, especially opioids, to manage pain that would be more effectively and safely treated with non-pharmacological approaches,” Alexander said. “This is a system that is designed with, and fosters, the idea that there is a pill for every ill...
- How IBM and the CDC are testing blockchain to track health issues like the opioid crisis (fastcompany.com)HIT Think How blockchain could solve 4 major problems in healthcare (healthdatamanagement.com)
IBM and CDC experts are hopeful that using a blockchain could help streamline long-running surveys that track patient symptoms and treatments...The Centers for Disease Control and IBM are collaborating on a blockchain-based system that could track public health issues like the ongoing opioid crisis...The new system, which IBM and the CDC’s National Center for Health Statistics have tested using simulated data, could make it easier for the CDC to survey medical providers about data like the reasons patients visit and the symptoms they display...Using the blockchain approach could make it easier to automatically collect the data, keep it secure, and log who’s accessed which parts of it...“There’s a lot of transparency that blockchain seems to offer to us,”...Blockchains are perhaps still most commonly associated with cryptocurrencies, where the secure digital ledgers are used to track who owns each unit of currency, with copies of the ever-growing chain of records automatically duplicated to anyone who wants them. But companies like IBM and Microsoft are exploring how the technology can be used in more traditional industries to sync up data like logs and transaction records between business associates, like health providers and the CDC. The automatic data replication can help maintain a reliable audit trail for more than just digital currency, they say...
- Superbugs now also becoming resistant to alcohol disinfectants (reuters.com)
Multidrug-resistant “superbugs” that can cause dangerous infections in hospitals are becoming increasingly resistant to alcohol-based hand sanitizers and disinfectants designed to hold them at bay...In a study of what the researchers described as a “new wave of superbugs”, the team also found specific genetic changes over 20 years in vancomycin-resistant Enterococcus, or VRE - and were able to track and show its growing resistance...efforts to tackle the rise of hospital superbugs such as VRE and MRSA, or methicillin-resistant Staphylococcus aureus, institutions worldwide have adopted stringent hygiene steps - often involving hand rubs and washes that contain alcohol...health authorities should try higher-alcohol concentrate products and renew efforts to ensure hospitals are deep cleaned and patients found to be carrying VRE infections are isolated...
- Study: With FDA Input, Compassionate Use Programs Appear to Work Well (ptcommunity.com)Availability of Investigational Medicines Through the US Food and Drug Administration’s Expanded Access and Compassionate Use Programs (jamanetwork.com)
When terminally ill Americans receive experimental medicines through so-called “compassionate use” programs, they typically only get these drugs after extensive tests for safety and effectiveness, a recent study suggests...This means that sufficient evidence of safety and effectiveness has been generated, ensuring that terminally ill patients are not being exposed to therapies that are unlikely to be beneficial or unsafe...this...suggests that companies and the FDA are providing expanded access to experimental therapies in a responsible way that protects patients and the public health...This system may change under The Right to Try Act of 2017, which would let companies decide whether to give patients experimental therapies without any input from the FDA...The chances of a drug not helping a patient or causing serious harm are higher when medicines are earlier in development, Paul Beninger, MD, MBA, of Tufts University School of Medicine...This is much more likely with Right to Try than with the current compassionate use system…
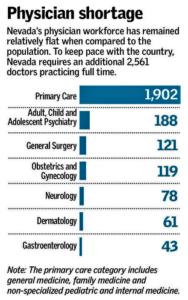
- Nevada again lands at bottom of national health-care ranking (reviewjournal.com)Nevada treading water despite efforts to address doctor shortage (reviewjournal.com)
Despite improvements in several areas, Nevada is once again listed at the bottom of a new national health care ranking...Nevada ranked 50th nationwide in a review of more than 100 population-level and patient-level measures published by the Agency for Healthcare Research and Quality. Only the District of Columbia scored lower overall than the Silver State...“There (are) actually areas where Nevada does fairly well — chronic disease care, diabetes, heart failure, stroke...Access to care remains a big problem. Nevada’s physician workforce hasn’t kept up with the state’s ballooning population, causing a doctor shortage...issues in accessing care, including getting an appointment with a primary care physician, having enough time in a doctor’s office and finding a specialist.
- How Can Medicare Save A Billion Bucks? Use Generics (ptcommunity.com)Medicare Spending on Brand-name Combination Medications vs Their Generic Constituents (jamanetwork.com)
In 2016, the cost difference between the amount that the Medicare Part D drug program spent on brand-name combination medications and the estimated cost for generic constituents for the same number of doses was $925 million, according to an investigation in the Journal of the American Medical Association...a retrospective analysis of Medicare drug spending from 2011 through 2016, looked at 29 brand-name oral combination medications, separated into three categories: medications that were available in generic form at identical doses; generic medications available at different doses; and therapeutically equivalent generic substitutes. The savings for all three categories was in the millions of dollars, with the largest discrepancy (an estimated $471 million) seen with the therapeutically equivalent generic substitutes. The authors also found that for the 10 most costly combination products that were available throughout the entire study period, the listed Medicare spending could have been an estimated $2.7 billion lower if the generic constituents had been prescribed.
- Doctors say pharmaceutical firms are top cause of high medical costs (healthcarefinancenews.com)
...high healthcare costs, most physicians claim they are not the ones to blame and instead pinpoint pharmaceutical and insurance companies, according to a new survey from University of Utah Health...members of the New England Journal of Medicine Catalyst Insights Council, who are clinicians, clinical leaders and executives involved with healthcare delivery...overwhelmingly believe pharmaceutical firms, followed closely by insurance companies, hospitals and health systems, have the biggest impact on costs...Other findings revealed by the survey showed that 86 percent believe physicians are not adequately trained to even discuss the cost of care, and 64 percent say there is not enough time to discuss the cost of treatments with patients. And 90 percent believe healthcare costs are too confusing for patients, while 78 percent feel the necessary tools are not available for patients to estimate those costs.
- FDA Previews Patient-focused Report on Opioid Use Disorder (raps.org)
The US Food and Drug Administration offered a sneak peek...into a new report on the impact of opioid use disorder (OUD) from the perspectives of about 155 patients...Preliminary results highlighted...offer insights into the impact of OUD on patients’ day-to-day lives to aid industry and FDA staff in the push for patient-focused drug development...The input can serve FDA and others in identifying specific areas of unmet needs for patients with OUD, support advice on medical product development programs and facilitate greater appropriate use of approved medications and how these are evaluated...The results highlight the impacts of OUD, a sensation called “craving,” treatment goals, as well as benefits and downsides of and the current barriers to medication-assisted treatment (MAT)...Benefits to MAT that patients reported include reducing the “euphoric rush,” as well as being able to “arrest the cravings and compulsiveness” and regain control of their life. In contrast, downsides relate to “intolerable or bothersome” side effects and the need to stay on MAT long-term. MAT also reportedly fails to address underlying issues, such as anxiety and pain...On challenges to MAT, participants cited a lack of access due to long wait times to obtain medication and strict requirements to remain in a program, among other issues. They also cited unpleasant experiences at MAT facilities, stigma, the intensity of withdrawals, addressing comorbid pain or mental health needs and a self-recognized need to come to terms with OUD.
- Antianxiety drugs — often more deadly than opioids — are fueling the next drug crisis in US (cnbc.com)
Today more than 40 million adults in the United States suffer from anxiety, and it is the most common mental illness in the United States...Overdose deaths involving benzodiazepines — such as Xanax, Librium, Valium and Ativan, drugs commonly used to treat anxiety, phobias, panic attacks, seizures and insomnia — have quadrupled between 2002 and 2015...The trend is being fueled by a 67 percent rise in prescriptions...The market for...(benzodiazepines)...is expected to reach $3.8 billion in the U.S. by 2020...many mental health experts are sounding the alarm, claiming that benzodiazepine addiction is an epidemic as frightening and serious as the opioid crisis...
- Evidence to support ‘breakthrough’ drugs often very limited: study (reuters.com)
The 46 medicines given approval through 2017 as part of the Food and Drug Administration’s Breakthrough Therapy program have often been sent to patients without a large double-blind study, direct measurement of benefit, or comparison with a placebo or existing treatment, according to a new analysis...“FDA approval of these breakthrough therapies is generally based on shorter and smaller clinical trials than those that support FDA approval of non-breakthrough therapy drugs,” coauthor Dr. Joseph Ross....expediting drug approvals raises concerns that important safety or effectiveness information will be missed, potentially heightening risk of patient harm...The study, published in the Journal of the American Medical Association, did not examine whether the drugs were actually as effective and safe as pre-approval testing suggested. In some cases the FDA doesn’t require such post-marketing studies for “breakthrough” drugs...people, when they hear ‘breakthrough designation,’ assume the ‘breakthrough’ is based on great clinical trial evidence but it’s actually more of an expectation that this is promising...My expectation is that this is what the public and clinicians (and Congress!) wants - more novel therapies coming to market as quickly as is reasonably possible, while still assuring drug safety and efficacy...questions persist on whether a larger trial will produce a different result, whether the benefit will fade as treatment continues, whether a drug will prove to be dangerous over the long term and whether a drug will really make a difference in long-term quality of life or survival...