- Do links count? FDA to re-examine online drug ads (medcitynews.com)
It’s back to the drawing board for the FDA...After years of slow reaction to pharma marketing online, the agency announced this week a new research initiative to better understand how consumers process short-form posts and ads...the FDA is trying to determine whether links in tweets and Google ads can independently convey all the necessary information about product risk...Under current guidelines, drug companies are required to balance the information they provide in character-space-limited posts. That means for a typical 140-character tweet, at least 70 characters must be dedicated to explaining risks and side effects...Regulatory change could be good news for pharma marketers, who have for a long time sought clarity on what they can and cannot do...
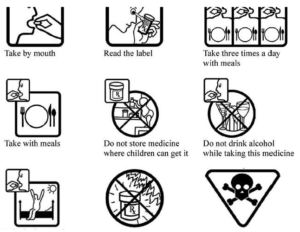
- New research reveals pictograms help seniors understand medication instructions (medicalxpress.com)
Nine different pharmaceutical pictograms that could help older people understand written medical information...Simple images designed to convey information about prescription drugs could help save lives and reduce the economic burden of non-adherence to treatment. New research...shows that including pictograms on written medication instructions helps seniors take their drugs correctly...Patients with multiple prescriptions can easily get confused and take the wrong medication, leading to hospitalization and even death…pictograms on a prescription drug label does help older people understand medical information and instructions. The pictograms provided information such as "take with meals" or "do not leave in direct sunlight" and warnings such as "poison" and "do not leave near children."...This not only prevents accidental overdose, it relieves some of the pressure that our aging population is putting on the health service by avoiding preventable tragedies...
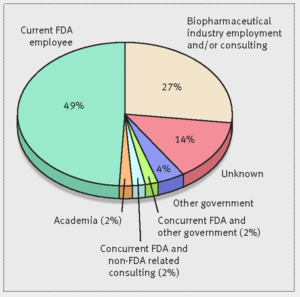
- Many ex-FDA drug reviewers take ‘revolving door’ to pharma industry (medcitynews.com)Future jobs of FDA’s haematology-oncology reviewers (bmj.com)
More than a quarter of the Food and Drug Administration employees who approved cancer and hematology drugs from 2001 through 2010 left the agency and now work or consult for pharmaceutical companies…Dr. Vinay Prasad, a hematologist-oncologist and assistant professor at Oregon Health and Science University, sought to understand the so-called "revolving door" between the FDA and the pharmaceutical industry, which he said is often discussed but hadn’t been quantified...Going to work for industry after leaving the FDA is not inherently bad, but it does raise some questions...If you know in the back of your mind that your career goal may be to someday work on the other side of the table, I wonder whether that changes the way you regulate...Are you more likely to give [companies] the benefit of the doubt? Are you less likely to beat them up hard over [using bad comparisons in drug studies]?...
- Report Finds Orphan Drug Pricing Increased More With Higher Off-Label Use (morningconsult.com)
Almost half of a special group of drugs, defined as those developed to treat rare diseases, were used for other purposes, according to a new report by America’s Health Insurance Plans. The greatest price increases among this group of drugs — called orphan drugs — were for those used for purposes other than treating the rare diseases they were developed to target...The insurers’ report charged this behavior is yet another example of drug companies potentially gaming the system for profit, the latest development in the rhetorical war between insurers and drug companies over drug costs...Orphan drugs, long thought to be treating so few patients each year that they did not present a reimbursement challenge for payers, are now being more closely scrutinized by public and private payers…study found that of its sample of 46 orphan drugs...47 percent of the drugs’ usage was for non-orphan purposes...
- Walter Reed starts testing Zika vaccine in humans, joining Inovio, NIAID jabs in the clinic (fiercepharma.com)
In a move that illustrates the stepped-up pace of Zika vaccine development, the Walter Reed Army Institute of Research is moving into human testing for its candidate, which uses an inactivated virus to spur immunity to the disease...It's the third Zika-fighting shot to step into the clinic...after Inovio Pharmaceuticals...and the NIH…Walter Reed plans to enroll 75 adults in a Phase I trial of that Zika purified inactivated virus (ZPIV) vaccine...To bolster its Zika effort, the institute has brought in other experts: It partnered with flavivirus vets at Sanofi Pasteur and Brazil’s Fiocruz. The collaborative effort has additional governmental assistance from the National Institute of Allergy and Infectious Diseases, and the Biomedical Advanced Research and Development Authority is contributing funding.
- Those risk disclosures on drug websites? People don’t read them, study says (fiercepharma.com)
Consumers don’t always read the risk information on branded drug websites--even though they say they do, according to new eye-tracking research from the University of Tennessee. Risk disclosures are a hot-button issue in pharma marketing, with some critics accusing brands of downplaying the risks and marketers contending that they follow the rules laid out by the FDA...The key finding? Even though 80% of the participants said they read half or more of the website information, they actually read much less than that and had limited recall of the drug’s risks. Further, the study found consumers focused on the drug’s benefit and generally ignored the risks... Mariea Hoy, who fielded the research...presented the findings to the FTC and has shared the same information with the FDA, also offered some suggestions on how to get people to read more drug risk information, Pharma companies should try to identify why people aren’t reading the risks, she said. Are they trying to avoid negative information? Do they think they already know the risks? Do they discount the risks for themselves, with the it-won’t-happen-to-me mindset known as "optimum bias?"...
- Diabetes drugs are badly needed, but rarely make it to market (statnews.com)
Diabetes may be a widespread disease for which millions of people need treatment, but a new analysis finds that developing new medicines has been a risky proposition for drug developers...How so? Here are a few key findings:
- Only 1 in 13 investigational diabetes drugs that entered clinical testing from 1995 to 2007 ultimately received regulatory approval, compared with 1 in 8 for all investigational drugs…
- The likelihood that a diabetes drug entering clinical testing would make it to late-stage testing was about 13 percent...
- the most important challenge for drug makers...continues to be the regulatory approval process, which has grown more demanding in the wake of a controversy in 2008 over the heart risks of a widely used diabetes drug.
- so-called first-in-class approvals for diabetes — which refers to new types of medicines — represented almost 30 percent of all FDA approvals.
- of all new diabetes drugs that were approved by the FDA from 1995 to 2015, 15 percent received a so-called priority review designation...
- The 61 diabetes and other endocrine drugs approved from 1995 to 2015 accounted for 10 percent of all new medicines approved by the FDA during that time...
- Physicians exhibit knowledge gap with biosimilars (chaindrugreview.com)
Though the vast majority of specialty physicians know what biosimilars are, their knowledge about these emerging medications falls short, according to a survey by the Biosimilars Forum...Of 1,201 U.S. physicians polled, 76.8% had heard the term biosimilars within the previous month...Yet respondents exhibited five key gaps in knowledge: defining biologics, biosimilars and biosimilarity; understanding the biosimilar approval process and the Food and Drug Administration’s use of totality of evidence to assess biosimilars; appreciation that biosimilars’ safety profile is expected to be the same as that of the originator biologic; understanding how the FDA makes decisions for extrapolation of indications; and defining interchangeability and the related rules regarding pharmacy-level substitution...With four biosimilars approved by the FDA and more than 60 in development, the survey highlights the need for greater biosimilars education for physicians and health care professionals...
- What experimental drug? Most companies don’t post compassionate use policies (statnews.com)
As patients clamor for greater access to experimental medicines, a survey released Tuesday finds that just 19 percent of 100 drug makers publicly post policies about their programs for obtaining these drugs, which are known as compassionate use. Moreover, only one of those companies posted information about specific procedures for making requests...The findings underscore arguments by a growing number of patient advocacy groups and lawmakers that the process for gaining access to experimental medicines is difficult, a complaint that has generated criticism of the Food and Drug Administration and sparked social media shaming campaigns of some companies...But other say the frustration directed at the FDA is misplaced. That’s because drug makers are actually the final gatekeepers and may deny requests in order to meet strict criteria needed to win FDA approval for a medicine...The FDA should clearly state in policy how adverse events under compassionate use will affect the ongoing clinical trial process for companies. While the FDA claims that this is a rare occurrence, the reality is that the FDA’s failure to issue clear policy leaves companies in a precarious position of jeopardizing their investments. If companies had clarity from the FDA about how offering drugs under the compassionate use program would impact ongoing trials, they’d be more likely to make their policies public and, possibly, to participate.
- Too many treatment guidelines are written by experts with financial conflicts, study finds (statnews.com)
Physicians typically rely on treatment guidelines issued by medical associations, but a new study finds that many experts involved in assembling these guidelines in Canada have financial ties to drug makers. And the study authors recommend that medical societies implement tougher disclosure rules to avoid undermining clinical decisions...researchers...examined 400 financial conflicts of interest statements in connection with the guidelines published in 2012 and 2013...found that relationships with drug makers varied...75 percent of the disclosures, at least one guideline author revealed such a relationship (conflicts of interest) and in 21 percent of the guidelines, all of the authors disclosed a conflict with drug companies...These guidelines...provide specific drug treatment recommendations that are considered to be authoritative regarding doctors・ treatment decisions for their patients...Clinical practice guidelines are also widely distributed by medical associations. Therefore, the financial relationships held by the physician-authors of these guidelines is an important step towards analyzing their choices of drug recommendations...