- Drugmakers open new year with price increases on dozens of medicines (biopharmadive.com)
Several major drugmakers, including Allergan, Bristol-Myers Squibb and Eli Lilly, increased prices on a number of their drugs Jan. 1, holding with typical industry practice despite continuing scrutiny of rising pharmaceutical costs...While raising prices in January has become the norm for pharma, this year has become a test of whether recent criticism by lawmakers and the Trump administration would spur companies to hold off on increases. Data cited by Raymond James suggests this could be playing out somewhat: Drugmakers overall have instituted fewer price hikes from Dec. 1 through Jan. 1 than the same period last year, according to a recent note from the investment bank...
- Judge blocks Trump administration cuts to 340B hospital drug-discount program (statnews.com)
A federal judge has blocked a Trump administration policy that reduces payments to hospitals under a drug discount program, ruling...that the government overstepped its authority in an attempt to address the high cost of prescription medications...The decision is a win for the 2,000-plus hospitals participating in the program, known as 340B, most of which serve large numbers of low-income patients...In 2017, the Department of Health and Human Services reduced its reimbursements for some drugs by about 28 percentage points...
- The valsartan carcinogen mess taught pharma a surprise manufacturing lesson. Will 2019 bring more? (fiercepharma.com)
Industry wisdom is that there is nothing new to learn about tablet making. Drugmakers have been doing it essentially the same way for more than 100 years. But that idiom was turned on its head this year when the FDA learned suspected carcinogens could be formed in “sartan” drugs from a specific sequence of manufacturing steps and chemical reactions—and that the U.S. drug supply had been riddled with them for years...The initial discovery of one of the impurities, N-nitrosodimethylamine, came this summer at a U.S. drug manufacturer that had used valsartan API from China’s Zhejiang Huahai Pharmaceutical...the FDA has since learned that the impurities were found in the APIs of other drugmakers, including Aurobindo and Mylan, and in finished products from Sandoz, Teva and others...
- Novartis unit teams up with medical marijuana producer, marking milestone for pharma (statnews.com)
Medical marijuana has stepped onto the global stage...In what appears to be a first for marijuana and a major pharmaceutical company, a Novartis subsidiary and a Canadian producer of medical cannabis products have agreed to market them around the world...Tilray Canada announced the agreement...Under the deal...Tilray and Sandoz will work together to supply and distribute non-smokable medical cannabis products (co-branding eight oil and capsule medical cannabis products)...the two companies will target. Tilray now sells its products in 12 countries and has operations in Canada, Australia, New Zealand, Germany, Portugal, and Latin America...
- What will 2019 bring for science and medicine? We asked the experts (statnews.com)
It has been a tumultuous year for science and medicine...We asked a whole host of experts — scientists, CEOs, policymakers, and professors — to weigh in on what themes they expect to see emerge in the next 12 months.
- We’re getting closer to a universal flu vaccine
- The CRISPR story is just getting started …
- And so is the focus on China.
- The opioid crisis isn’t slowing down, either.
- Speaking of cannabis (and psychedelics) … it’s only heating up.
- Cancer research will increasingly focus on organoids.
- You’ll get more control of your health data.
- Broadly, though, expect a reckoning in the AI space.
- None of this will keep prices down.
- We’ll get a clearer picture on antibiotic resistance threats.
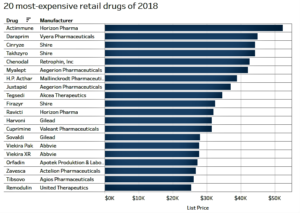
- Pharmacies may have to change to stay relevant. - The 20 most expensive pharmacy drugs in 2018, featuring names big and small (fiercepharma.com)
More and more, specialty drugs carrying eye-popping price tags are winning FDA approvals. While more are certainly on the way—look at Novartis' recent presentation that a gene therapy could be worth millions per patient—analysts at drug pricing website GoodRx have tallied up the most expensive pharmacy-dispensed drugs in the U.S. per month as of November...Some are marketed by small pharma companies such as Vyera Pharmaceuticals, while top biotechs and pharmaceutical companies market others. Some have seen controversy, while others are lesser known. Rare disease meds make up much of the list, while some treatments such as those for hepatitis C treat diseases that affect millions of people...
- This Week in Managed Care: December 21, 2018 (ajmc.com)
Laura Joszt, Managing Editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- Crash and burn: Why three biotechs failed in 2018 (statnews.com)
There is an appointed time for everything — including in biotech. There is a time to start a company, a time to wind it down, a time to go public, and a time to delist the stock...For every hopeful biotech toiling for decades before finally realizing a profit, there are countless others that run out of money or bet on a product that never delivers...STAT took a look at some of the biopharma and biotech companies that wound down their operations over the course of 2018 and what those companies’ executives are doing now.
Orexigen Therapeutics
Argos Therapeutics
Sancilio Pharmaceuticals - Punishing Patients Won’t Reduce Opioid Deaths (reason.com)
Barbara McAneny, president of the American Medical Association, recently described a patient with metastatic prostate cancer who tried to kill himself after he could not get the medication he was prescribed for bone pain because...his insurer...denied coverage...my patient nearly died of an underdose...McAneny was talking about the suffering caused by government pressure to reduce opioid prescriptions, which has led to denials of treatment and arbitrary dose reductions...A Medicare rule that take effect on January 1 will compound that problem...
- Congressional report: Drug companies, DEA, failed to stop flow of millions of opioid pills (washingtonpost.com)Committee Report Details Alleged Opioid-Dumping in West Virginia (energycommerce.house.gov)Red Flags and War ning Signs Ignored: Opioid Distribution and Enforcement Concerns in West Virginia (energycommerce.house.gov)
A report from the majority staff of the House Energy and Commerce Committee found that distributors, which fulfill orders for prescription drugs to pharmacies, failed to conduct proper oversight of their customers by not questioning suspicious activity and not properly monitoring the quantity of painkillers that were being shipped to individual pharmacies...The committee also found that the DEA did not properly use a database that aims to monitor the flow of powerful prescription painkillers from manufacturers to sellers, something that could have allowed federal agents — in real time —...The agency also curtailed enforcement of distributors...and infighting inside the agency affected the way cases were handled...